Textile Performance of Polyester, Nylon 6 and Acetate Fabrics Treated with Atmospheric Pressure Plasma Jet Transaction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
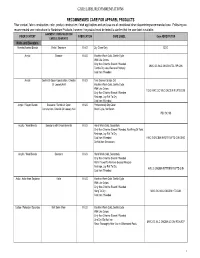
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Analysis of the Polyester Clothing Value Chain to Identify Key Intervention Points for Sustainability Cristina Palacios‑Mateo* , Yvonne Van Der Meer and Gunnar Seide
Palacios‑Mateo et al. Environ Sci Eur (2021) 33:2 https://doi.org/10.1186/s12302‑020‑00447‑x REVIEW Open Access Analysis of the polyester clothing value chain to identify key intervention points for sustainability Cristina Palacios‑Mateo* , Yvonne van der Meer and Gunnar Seide Abstract Clothing is one of the primary human needs, and the demand is met by the global production of thousands of tons of textile fbers, fabrics and garments every day. Polyester clothing manufactured from oil‑based polyethylene tereph‑ thalate (PET) is the market leader. Conventional PET creates pollution along its entire value chain—during the produc‑ tion, use and end‑of‑life phases—and also contributes to the unsustainable depletion of resources. The consumption of PET garments thus compromises the quality of land, water and air, destroys ecosystems, and endangers human health. In this article, we discuss the diferent stages of the value chain for polyester clothing from the perspective of sustainability, describing current environmental challenges such as pollution from textile factory wastewater, and microfbers released from clothing during the laundry cycle. We also consider potential solutions such as enhanced reuse and recycling. Finally, we propose a series of recommendations that should be applied to polyester clothing at all stages along the value chain, ofering the potential for meaningful and efective change to improve the environ‑ mental sustainability of polyester textiles on a global scale. Keywords: PET, Textiles, Value chain, Environmental sustainability, Microfbers, Pollution, Recycling, Life cycle Introduction with 2019 estimates in Germany suggesting an average Te global volume of fber production for textile manu- lifetime of only 4.4 years [5]. -

DID YOU KNOW? PET (Polyethylene Terephthalate) Is Actually Polyester. When PET Is Used for Bottles, Containers and Other
355 Lexington Ave., Suite 1500 ▪ New York, NY 10017 ▪ www.PETresin.org DID YOU KNOW? Little-Known Facts about PET Plastic . PET (polyethylene terephthalate) is actually polyester. When PET is used for bottles, containers and other applications, it is called PET or PET resin. When PET is used as a fiber, it is typically called polyester. The PET bottle was invented by Nathaniel C. Wyeth, a DuPont engineer and brother of American painter Andrew Wyeth. The patent was issued to Wyeth in 1973 and assigned to DuPont. According to the EPA, recycling one pound of PET bottles (that’s about 10 two-liter soda bottles) saves approximately 26,000 BTUs of energy. PET bottles and the sun are helping millions of people in developing countries obtain potable water. Using a system called SODIS (solar water disinfection), inhabitants set water-filled PET bottles in the sun for several hours or days – depending on how much sunlight is available – as a simple but effective means of destroying disease-causing bacteria and gaining safe drinking water. More than 1.5 billion pounds of used PET bottles and containers are collected in the U.S. each year for recycling. PET is the most recycled plastic in the U.S. and the world. A single-serve PET bottle (0.5 liter) is strong enough to hold 50 times its weight in water. Chemists keep finding new ways to make PET lighter without losing any strength. A 2-liter PET bottle that weighed 68 grams in 1980 now weighs as little as 42 grams. The average weight of single-serve 0.5 liter PET water bottle is now 9.9 grams, nearly half of what it weighed in 2000. -

Handle and Comfort Characteristics of Cotton Core Spun Lycra and Polyester/ Lycra Fabrics for Application As Blouse Nirmala Varghese and G
Volume 8, Issue 4, Spring2014 Handle and Comfort Characteristics of Cotton Core Spun Lycra and Polyester/ Lycra Fabrics for Application as Blouse Nirmala Varghese and G. Thilagavathi Department of Apparel & Fashion Technology PSG College of Technology Tamil Nadu, India ABSTRACT Stretchable plain woven fabrics are developed with 60s cotton core spun Lycra and polyester Lycra twisted yarn (91D) in weft with cotton, silk and polyester in warp. The influence of fabric specifications on thermal comfort, low stress mechanical properties, primary and total hand value is analyzed. The results indicated that cotton core spun Lycra woven with polyester warp provided higher value of thermal conductivity, air permeability and water vapor permeability. The woven stretch fabrics produced with polyester warp, cotton core spun Lycra and polyester Lycra in weft has excellent aesthetic and drape properties. These fabrics have higher tensile resilience, tensile strain, lower shear rigidity, superior primary hand and a higher total hand value of 4.08 and 3.93 respectively and are best suitable for women’s blouse. Keywords: Cotton core spun Lycra, polyester/Lycra, woven stretch, fabric handle, sari blouse Introduction Lycra and polyester/lycra twisted yarn. Several studies have been carried out Stretch is often the most important investigating the physical properties of core property of textiles that contribute to wearer spun yarn, cotton/lycra and properties of the comfort. The stretch yarn and fabrics are fabrics containing different rates of spandex widely used and preferred by the people in yarns. Core spun cotton/spandex shows high the last few years all over the world because resiliency property than 100% cotton yarns, of their stretch ability and shape retention due to its soft and rubbery isocyanate properties. -
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Blending Cotton and Polyester Fibers
BLENDING COTTON AND POLYESTER FIBERS-- EFFECTS OF PROCESSING METHODS ON FIBER DISTRIBUTION AND YARN PROPERTIES A THESIS Presented to The Faculty of the Graduate Division by Nelson Ping Ching Chao In Partial Fulfillment of the Requirements for the Degree Master of Science in Textiles Georgia Institute of Technology September, 1963 3^ 12,^ ^ BLENDING COTTON AND POLYESTER FIBERS- EFFECTS OF PROCESSING METHODS ON FIBER DISTRIBUTION AND YARN PROPERTIES Approved! "—xy '—17 Date Approved by Chairman: ^^ysu^.^1^ ^h^ R^^ li ACKNOWLEDGMENTS The author wishes to express his sincere gratitude and apprecia tion to Dr. James L. Taylor, Director of the A. French Textile School, £or granting him a graduate assistantship and a Celanese fellowship which made this study possible; to Professor R. K. Flege, his thesis advisor, and to Professor R. L. Hill, both of the A. French Textile School, for their advice and recommendations; and to Dr. Joseph J. Moder of the School of Industrial Engineering for his assistance with the statistical aspects of the experiment. He also extends his thanks to Mr. R. C. Freeman, technician of the A. French Textile School, for his kind assistance. iii TABLE OF CONTENTS Page ACKNOWLEDGMENTS ii LIST OF TABLES iv LIST OF ILLUSTRATIONS vii SUMMARY vill Chapter I. INTRODUCTION I Some Aspects of Blending Fibers Stateioent of the Problem II. INSTRUMENTATION AND EQUIPMENT 11 Raw Materials Used Processing Equipment Testing Equipment III. PROCEDURE 13 Processing Blended Yarn Physical Tests Tests for Uniformity Tests for Breaking Strength and Elongation of Yarn Examination of Fiber Distribution Analyses of Data IV. DISCUSSION OF RESULTS 24 V. -

Evaluation of the Physical and Diagnostic Nature of Swabs W
EVALUATION OF THE PHYSICAL AND DIAGNOSTIC NATURE OF SWABS W. Nugent, M.D.1, M.J. Russell1 S. Beck 2, and G.P. Leonardi, Ph.D.1,3 NASSAU 1 M44 Department of Pathology, Nassau University Medical Center, East Meadow, NY ; UNIVERSITY Department of Biology, Nassau Community College, Garden City, NY2 and Sunrise Medical Laboratories, Hauppauge, NY3 medicalcenter Abstract: Conclusions: Proper specimen collection is the single most important factor in obtaining a reliable and and reweighed. Following addition of water (1.0ml), tubes were shaken at high speeds ß Fiber confi guration and size of the nylon-fl ocked swab allowed for greater accurate diagnostic result. Swabs are often employed to collect specimens for a variety for 1 minute, followed by centrifugation using a table-top Hermle Z180M centrifuge absorbance than the actual nylon material itself. of microbiologic assays. A novel, nylon-tipped, “fl ocked“ swab (Copan, Inc; Corona, CA) (Universal Labnet Co., Edison, NJ) for 5 minutes at maximum speed. Unabsorbed water FIG. 1 FIG. 2 has recently become commercially available. Unlike conventional swabs in which fi bers ß There are numerous other factors, not presently examined, which can aff ect was removed by decanting and wiping the inner tube surface with bibulous paper, swab performance. are woven on a shaft material, fl ocked swabs have a “one piece” construction with nylon and the tube was reweighed. This exercise was repeated twice. fi bers radiating from the shaft tip. This construction may enhance the amount of specimen ß Nevertheless, our data suggests that using of nylon-fl ocked swabs would available for diagnostic testing. -

Fabric Supplier List
FABRIC SUPPLIER LIST CANADA Kendor Textiles Ltd 1260 Cliveden Ave Delta BC V3M 6Y1 Canada 604.434.3233 [email protected] www.kendortextiles.com Fabrics Available: Fabric supplier. Eco-friendly. Organic. Knits: solids, prints, yarn dyes and warp. Wovens: solids and yarn dyes. End Use: activewear, bottomweights, medical, lingerie, childrenswear, swimwear, rainwear, skiwear and uniform. Natural & eco items include cottons, bamboo's, modals, linens, hemps, organic cottons & organic linens. Technical items include waterproof/breathable soft shells, antibacteric & wicking polyester & recycled polyesters. Is a proud representative of the British Millerain line of waxed cottons and wools, and are able to provide custom souring. Minimums: Carries stock. In-stock minimum: 5 yards/color. Minimum order for production: 10 yards/color. Gordon Fabrics LTD #1135-6900 Graybar Rd. Richmond BC Canada 604.275.2672 [email protected] Fabrics Available: Fabric Supplier. Importer. Jobber. Carries stock. Knits & Wovens: solids, prints, yarn dyes and novelties. End Use: activewear, borromweights, eveningwear/bridal, medical, lingerie and childrenswear. Minimums: In stock minimum 1 yard. Minimum order for production varies. StartUp Fashion Supplier List 2016 – Page 1 CHINA Ecopel (HX) Co., Ltd. China +86 216.767.9686 www.ecopel.cn Fabrics Available: Fake fur and leather garments. End Uses: Childrenswear, Menswear, Other, Womenswear. Minimums: Min. order 50-100 m Hangzhou New Design Source Textile Co., Ltd. China +86 057.182.530528 Fabrics Available: Knits, Polyester/Man-Made, Prints. End Uses: Juniors Fashion, Menswear, Womenswear. Minimums: Min order 50 m. Nantong Haukai Textile Co., Ltd. China +86 513.890.78626 www.huakaitex.com Fabrics Available: Cotton, Linen. End Uses: Corporatewear/Suiting, Menswear, Womenswear. -

Geiger Textiles Price List
Price List and Specification Guide EFFECTIVE JUNE 2021 02 Textiles 84 Price Groups 85 Custom Finishes 86 Warranty 87 Additional Information 88 Maintenance Guidelines 800.456.6452 geigertextiles.com © 2021 Geiger 1 Allusion DESIGNED BY BASSAMFELLOWS APPLICATION Seating CONTENT 60% Alpaca, 27% Wool, 13% Nylon BACKING Cotton WIDTH 56" REPEAT None ABRASION 95,000 Cycles, Martindale* FLAMMABILITY CA TB 117-2013 WEIGHT 25.2 Oz Per Linear Yard 1GS01 Moonlight 1GS02 Pearl Gray 1GS03 Platinum ORIGIN Italy ENVIRONMENTAL SCS Indoor Advantage™ Gold Contains Bio-Based Materials FR Chemical Free Prop 65 Chemical Free REACH Compliant Healthier Hospitals Compliant Living Future Red List Compliant WELL Building Standard Compliant 1GS04 Smoky Taupe 1GS05 Camel 1GS06 Swiss Red MAINTENANCE S – Clean with Mild, Dry Cleaning Solvent CUSTOM FINISHES Alta™ Plush PRICE GROUP 8 NET PRICE $135 Per Yard *Abrasion test results exceeding ACT Performance Guidelines are not an indicator of product lifespan. Multiple factors affect fabric durability 1GS07 Chestnut 1GS08 Deep 1GS09 Navy Brown Cerulean and appearance retention. 1GS10 Black Green 1GS11 Sterling 1GS12 Anthracite 800.456.6452 geigertextiles.com © 2021 Geiger 2 Alpaca Mohair DESIGNED BY SUSAN LYONS APPLICATION Seating CONTENT 100% Alpaca BACKING Cotton/Polyester WIDTH 54" REPEAT None ABRASION 40,000 Cycles, Martindale FLAMMABILITY CA TB 117-2013 WEIGHT 29.7 Oz Per Linear Yard 18510 Dune 18511 Trench 18512 Vicuna ORIGIN Belgium ENVIRONMENTAL SCS Indoor Advantage™ Gold Contains Bio-Based Materials FR Chemical -

NOLENVFAB Eviro Fabrics List
Our Products Reflect Stewardship Sustainable Textiles It’s easy to choose earth-friendly fabrics with National. At National Office Furniture, our textile offering includes fabrics with a reduced environmental impact. Choices include fabrics with significant recycled content, as well as PVC or chrome-free. Some bear the Terratex® name which signifies they are made of 100% recycled fiber and use a fabric production process that reduces waste and emissions. Others are made of Repreve® yarn, a recycled polyester yarn that uses the U Trust™ verification program which ensures that Repreve recycled fibers are traceable, transparent and certifiably sustainable. In addition, some fabrics are Green-e® certified, signifying that 100% of the electricity used to make the fabric is matched by Green-e® certified renewable energy certificates that support clean energy. Additionally, virtually all National in-line fabrics have no added flame retardant chemicals (FRs). Our alliance partners, Arc-Com, Stinson, Maharam, Momentum and Ultrafabrics, also offer upholstery choices that feature sustainable characteristics. For additional details, please refer to their websites. Seating Upholstery Contents Finish Backing Reduced Environmental Impact 50% Recycled Solution-Dyed Nylon Big City BLOCKaide™ Acrylic Recycled Content 50% Solution-Dyed Nylon Boardwalk 100% Pre-Consumer Recycled Polyester BLOCKaide™ SBR Latex Recycled Content Brisa® 100% Polyurethane None Rayon PVC-Free Brisa® Distressed 100% Polyurethane None Rayon PVC-Free 65% Pre-Consumer Recycled Polyester, -

A Study of Anti-Odor and UV-Protection Behavior on Silk and Its Polyester / Lyocell Mixed Fabric
Volume 9, Issue 2, Spring2015 A Study of Anti-odor and UV-protection Behavior on Silk and its Polyester / Lyocell Mixed Fabric J. Jeyakodi Moses1, A. Mariappan2, and K. Vellingiri3 1,2Department of Applied Science, PSG College of Technology 3Department of Fashion Technology, PSG College of Technology India [email protected] ABSTRACT Silk and lyocell are natural protein and cellulose polymeric materials. They are important in the fields of textile, apparel and garment. Polyester (PET) is one of the synthetic textiles with very good resistance towards chemical and microbial attacks. In this study it is decided to mix silk with polyester and lyocell. The fabric is then dyed with both natural dyes (kum, indigo, bar berry) and synthetic dyes (reactive dye (H), reactive dye (M) and Sulphur dye). This mixed fabric is compared with 100% silk for the k/s value, fastness properties, drapeability, thermal resistance, anti-odor and uv-protection behaviors. The silk mixed fabric gives very good results compared with the 100% silk fabric. Keywords: Silk, lyocell, k/s value, anti-odor, uv-protection Introduction from silkworm have two major protein The natural silk fiber protein is constituents. Core is made of fibroin protein synthesized by silk gland cells and stored which is surrounded by outer sericin protein4. in the lumen of the silk glands. Insects The core fibroin is made up of two chains mainly belong to two families, viz., known as heavy chain and light chain, mainly Saturnidae and Bombycidae, which spins constitute of alanine, tyrosine and glycine5,6. silk fiber. Bombyx mori belongs to Silk proteins are natural polymers and are Bombycidae produces a delicate twin biodegradable with reactive functional thread of silk fibroin, which is coated by a groups that open up possibility to be protective cover of sericin.