CAP Cancer Protocol Uterine Cervix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Reference Sheet 1
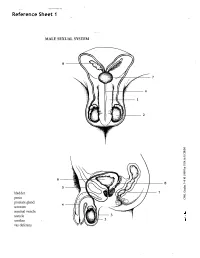
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Uterine Sarcomas: a Review
ARTICLE IN PRESS YGYNO-973334; No. of pages: 9; 4C: 3, 6 Gynecologic Oncology xxx (2009) xxx–xxx Contents lists available at ScienceDirect Gynecologic Oncology journal homepage: www.elsevier.com/locate/ygyno Review Uterine sarcomas: A review Emanuela D'Angelo, Jaime Prat ⁎ Department of Pathology, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Sant Antoni M. Claret, 167, 08025 Barcelona, Spain article info abstract Article history: Objective. Uterine sarcomas are rare tumors that account for 3% of uterine cancers. Their histopathologic Received 29 June 2009 classification was revised by the World Health Organization (WHO) in 2003. A new staging system has been Available online xxxx recently designed by the International Federation of Gynecology and Obstetrics (FIGO). Currently, there is no consensus on risk factors for adverse outcome. This review summarizes the available clinicopathological data Keywords: on uterine sarcomas classified by the WHO diagnostic criteria. Uterine sarcomas Methods. Medline was searched between 1976 and 2009 for all publications in English where the studied Leiomyosarcoma population included women diagnosed of uterine sarcomas. Endometrial stromal sarcoma fi Undifferentiated endometrial sarcoma Results. Since carcinosarcomas (malignant mixed mesodermal tumors or MMMT) are currently classi ed Adenosarcoma as metaplastic carcinomas, leiomyosarcomas remain the most common uterine sarcomas. Exclusion of Carcinosarcoma several histologic variants of leiomyoma, as well as “smooth muscle tumors of uncertain malignant potential,” frequently misdiagnosed as sarcomas, has made apparent that leiomyosarcomas are associated with poor prognosis even when seemingly confined to the uterus. Endometrial stromal sarcomas are indolent tumors associated with long-term survival. Undifferentiated endometrial sarcomas exhibiting nuclear pleomorphism behave more aggressively than tumors showing nuclear uniformity. -

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -

Ovarian Cancer and Cervical Cancer
What Every Woman Should Know About Gynecologic Cancer R. Kevin Reynolds, MD The George W. Morley Professor & Chief, Division of Gyn Oncology University of Michigan Ann Arbor, MI What is gynecologic cancer? Cancer is a disease where cells grow and spread without control. Gynecologic cancers begin in the female reproductive organs. The most common gynecologic cancers are endometrial cancer, ovarian cancer and cervical cancer. Less common gynecologic cancers involve vulva, Fallopian tube, uterine wall (sarcoma), vagina, and placenta (pregnancy tissue: molar pregnancy). Ovary Uterus Endometrium Cervix Vagina Vulva What causes endometrial cancer? Endometrial cancer is the most common gynecologic cancer: one out of every 40 women will develop endometrial cancer. It is caused by too much estrogen, a hormone normally present in women. The most common cause of the excess estrogen is being overweight: fat cells actually produce estrogen. Another cause of excess estrogen is medication such as tamoxifen (often prescribed for breast cancer treatment) or some forms of prescribed estrogen hormone therapy (unopposed estrogen). How is endometrial cancer detected? Almost all endometrial cancer is detected when a woman notices vaginal bleeding after her menopause or irregular bleeding before her menopause. If bleeding occurs, a woman should contact her doctor so that appropriate testing can be performed. This usually includes an endometrial biopsy, a brief, slightly crampy test, performed in the office. Fortunately, most endometrial cancers are detected before spread to other parts of the body occurs Is endometrial cancer treatable? Yes! Most women with endometrial cancer will undergo surgery including hysterectomy (removal of the uterus) in addition to removal of ovaries and lymph nodes. -

Echography of the Cervix and Uterus During the Proliferative and Secretory Phases of the Menstrual Cycle in Bonnet Monkeys (Macaca Radiata)
Journal of the American Association for Laboratory Animal Science Vol 53, No 1 Copyright 2014 January 2014 by the American Association for Laboratory Animal Science Pages 18–23 Echography of the Cervix and Uterus during the Proliferative and Secretory Phases of the Menstrual Cycle in Bonnet Monkeys (Macaca radiata) Uddhav K Chaudhari,1,* Siddnath M Metkari,2 Dhyananjay D Manjaramkar,2 Geetanjali Sachdeva,1 Rajendra Katkam,1 Atmaram H Bandivdekar,3 Abhishek Mahajan,4 Meenakshi H Thakur,4 and Sanjiv D Kholkute1 We undertook the present study to investigate the echographic characteristics of the uterus and cervix of female bonnet monkeys (Macaca radiata) during the proliferative and secretory phases of the menstrual cycle. The cervix was tortuous in shape and measured 2.74 ± 0.30 cm (mean ± SD) in width by 3.10 ± 0.32 cm in length. The cervical lumen contained 2 or 3 col- liculi, which projected from the cervical canal. The echogenicity of cervix varied during proliferative and secretory phases. The uterus was pyriform in shape (2.46 ± 0.28 cm × 1.45 ± 0.19 cm) and consisted of serosa, myometrium, and endometrium. The endometrium generated a triple-line pattern; the outer and central lines were hyperechogenic, whereas the inner line was hypoechogenic. The endometrium was significantly thicker during the secretory phase (0.69 ± 0.12 cm) than during the proliferative phase (0.43 ± 0.15 cm). Knowledge of the echogenic changes in the female reproductive organs of bonnet monkeys during a regular menstrual cycle may facilitate understanding of other physiologic and pathophysiologic changes. Ultrasound imaging is a noninvasive, atraumatic, and simple Materials and Methods method to assess various organs in humans and nonhuman pri- Animals and husbandry practices. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

Primary Immature Teratoma of the Thigh Fig
CORRESPONDENCE 755 8. Gray W, Kocjan G. Diagnostic Cytopathology. 2nd ed. London: Delete all that do not apply: Elsevier Health Sciences, 2003; 677. 9. Richards A, Dalrymple C. Abnormal cervicovaginal cytology, unsatis- Cervix, colposcopic biopsy/LLETZ/cone biopsy: factory colposcopy and the use of vaginal estrogen cream: an obser- vational study of clinical outcomes for women in low estrogen states. Diagnosis: NIL (No intraepithelial lesion WHO 2014) J Obstet Gynaecol Res 2015; 41: 440e4. LSIL (CIN 1 with HPV effect WHO 2014) 10. Darragh TM, Colgan TJ, Cox T, et al. The lower anogenital squamous HSIL (CIN2/3 WHO 2014) terminology standardization project for HPV-associated lesions: back- Squamous cell carcinoma ground and consensus recommendation from the College of American Immature squamous metaplasia Pathologists and the American Society for Colposcopy and Cervical Adenocarcinoma in situ (AIS, HGGA) e Adenocarcinoma Pathology. Arch Pathol Lab Med 2012; 136: 1267 97. Atrophic change 11. McCluggage WG. Endocervical glandular lesions: controversial aspects e Extending into crypts: Not / Idenfied and ancillary techniques. J Clin Pathol 2013; 56: 164 73. Epithelial stripping: Not / Present 12. World Health Organization (WHO). Comprehensive Cervical Cancer Invasive disease: Not / Idenfied / Micro-invasive Control: A Guide to Essential Practice. 2nd ed. Geneva: WHO, 2014. Depth of invasion: mm Transformaon zone: Not / Represented Margins: DOI: https://doi.org/10.1016/j.pathol.2019.07.014 Ectocervical: Not / Clear Endocervical: Not / Clear Circumferenal: Not / Clear p16 status: Negave / Posive Primary immature teratoma of the thigh Fig. 3 A proposed synoptic reporting format for pathologists reporting colposcopic biopsies and cone biopsies or LLETZ. Sir, Teratomas are germ cell tumours composed of a variety of HSIL, AIS, micro-invasive or more advanced invasive dis- somatic tissues derived from more than one germ layer 12 ease. -

Squamous Cell Carcinoma Arising in an Ovarian Mature Cystic Teratoma
Case Report Obstet Gynecol Sci 2013;56(2):121-125 http://dx.doi.org/10.5468/OGS.2013.56.2.121 pISSN 2287-8572 · eISSN 2287-8580 Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy Nae-Ri Yun1, Jung-Woo Park1, Min-Kyung Hyun1, Jee-Hyun Park1, Suk-Jin Choi2, Eunseop Song1 Departments of 1Obstetrics and Gynecology and 2Pathology, Inha University College of Medicine, Incheon, Korea Mature cystic teratomas of the ovary (MCT) are usually observed in women of reproductive age with the most dreadful complication being malignant transformation which occurs in approximately 1% to 3% of MCTs. In this case report, we present a patient with squamous cell carcinoma which developed from a MCT during pregnancy. The patient was treated conservatively without adjuvant chemotherapy and was followed without evidence of disease for more than 60 months using conventional tools as well as positron emission tomography-computed tomography following the initial surgery. We report this case along with the review of literature. Keywords: Dermoid cyst; Malignant transformation; Observation; Positron emission tomography-computed tomography Introduction An 18 cm solid and cystic left ovarian mass with a smooth surface and two small right ovarian cysts were detected re- The incidence of adnexal masses during pregnancy is 1% to sulting in a laparotomy at 13 weeks of gestation and left sal- 9% [1]. Mature cystic teratomas (MCT) are common during pingo-oophorectomy and right ovarian cystectomy (Fig. 1C). pregnancy with the most dreadful complication being ma- The report of the frozen section from both tissues revealed lignant transformation which occurs in approximately 1% to MCT. -

Pembrolizumab in Vaginal and Vulvar Squamous Cell Carcinoma: a Case Series from a Phase II Basket Trial Jefrey A
www.nature.com/scientificreports OPEN Pembrolizumab in vaginal and vulvar squamous cell carcinoma: a case series from a phase II basket trial Jefrey A. How 1, Amir A. Jazaeri 1, Pamela T. Soliman1, Nicole D. Fleming1, Jing Gong2, Sarina A. Piha‑Paul2, Filip Janku 2, Bettzy Stephen 2 & Aung Naing 2* Vaginal and vulvar squamous cell carcinoma (SCC) are rare tumors that can be challenging to treat in the recurrent or metastatic setting. We present a case series of patients with vaginal or vulvar SCC who were treated with single‑agent pembrolizumab as part of a phase II basket clinical trial to evaluate efcacy and safety. Two cases of recurrent and metastatic vaginal SCC, with multiple prior lines of systemic chemotherapy and radiation, received pembrolizumab. One patient had signifcant reduction (81%) in target tumor lesions prior to treatment discontinuation at cycle 10 following confrmed progression of disease with new metastatic lesions (stable disease by irRECIST criteria). In contrast, the other patient with vaginal SCC discontinued treatment after cycle 3 due to disease progression. Both patients had PD‑L1 positive vaginal tumors and tolerated treatment well. One case of recurrent vulvar SCC with multiple surgical resections and prior progression on systemic carboplatin had a 30% reduction in her target tumor lesions following pembrolizumab treatment with a PD‑L1 positive tumor. Treatment was discontinued for grade 3 mucositis after cycle 5. Pembrolizumab may provide some clinical beneft to some patients with vaginal or vulvar SCC and is overall safe to utilize in this population. Future studies are needed to evaluate the efcacy of pembrolizumab in these rare tumor types and to identify predictive biomarkers of response. -

Influence of Age on Histologic Outcome of Cervical Intraepithelial Neoplasia
www.nature.com/scientificreports OPEN Infuence of age on histologic outcome of cervical intraepithelial neoplasia during observational Received: 10 May 2017 Accepted: 6 April 2018 management: results from large Published: xx xx xxxx cohort, systematic review, meta- analysis Christine Bekos1, Richard Schwameis1, Georg Heinze2, Marina Gärner1, Christoph Grimm1, Elmar Joura1,4, Reinhard Horvat3, Stephan Polterauer1,4 & Mariella Polterauer1 Aim of this study was to investigate the histologic outcome of cervical intraepithelial neoplasia (CIN) during observational management. Consecutive women with histologically verifed CIN and observational management were included. Histologic fndings of initial and follow-up visits were collected and persistence, progression and regression rates at end of observational period were assessed. Uni- and multivariate analyses were performed. A systematic review of the literature and meta-analysis was performed. In 783 women CIN I, II, and III was diagnosed by colposcopically guided biopsy in 42.5%, 26.6% and 30.9%, respectively. Younger patients had higher rates of regression (p < 0.001) and complete remission (< 0.001) and lower rates of progression (p = 0.003). Among women aged < 25, 25 < 30, 30 < 35, 35 < 40 years, and > 40 years, regression rates were 44.7%, 33.7%, 30.9%, 27.3%, and 24.9%, respectively. Pooled analysis of published data showed similar results. Multivariable analysis showed that with each fve years of age, the odds for regression reduced by 21% (p < 0.001) independently of CIN grade (p < 0.001), and presence of HPV high-risk infection (p < 0.001). Patient’s age has a considerable infuence on the natural history of CIN – independent of CIN grade and HPV high- risk infection. -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

Clinical Radiation Oncology Review
Clinical Radiation Oncology Review Daniel M. Trifiletti University of Virginia Disclaimer: The following is meant to serve as a brief review of information in preparation for board examinations in Radiation Oncology and allow for an open-access, printable, updatable resource for trainees. Recommendations are briefly summarized, vary by institution, and there may be errors. NCCN guidelines are taken from 2014 and may be out-dated. This should be taken into consideration when reading. 1 Table of Contents 1) Pediatrics 6) Gastrointestinal a) Rhabdomyosarcoma a) Esophageal Cancer b) Ewings Sarcoma b) Gastric Cancer c) Wilms Tumor c) Pancreatic Cancer d) Neuroblastoma d) Hepatocellular Carcinoma e) Retinoblastoma e) Colorectal cancer f) Medulloblastoma f) Anal Cancer g) Epndymoma h) Germ cell, Non-Germ cell tumors, Pineal tumors 7) Genitourinary i) Craniopharyngioma a) Prostate Cancer j) Brainstem Glioma i) Low Risk Prostate Cancer & Brachytherapy ii) Intermediate/High Risk Prostate Cancer 2) Central Nervous System iii) Adjuvant/Salvage & Metastatic Prostate Cancer a) Low Grade Glioma b) Bladder Cancer b) High Grade Glioma c) Renal Cell Cancer c) Primary CNS lymphoma d) Urethral Cancer d) Meningioma e) Testicular Cancer e) Pituitary Tumor f) Penile Cancer 3) Head and Neck 8) Gynecologic a) Ocular Melanoma a) Cervical Cancer b) Nasopharyngeal Cancer b) Endometrial Cancer c) Paranasal Sinus Cancer c) Uterine Sarcoma d) Oral Cavity Cancer d) Vulvar Cancer e) Oropharyngeal Cancer e) Vaginal Cancer f) Salivary Gland Cancer f) Ovarian Cancer & Fallopian