Allergen Extracts Prior Authorization Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pindolol of the Activation of Postsynaptic 5-HT1A Receptors
Potentiation by (-)Pindolol of the Activation of Postsynaptic 5-HT1A Receptors Induced by Venlafaxine Jean-Claude Béïque, Ph.D., Pierre Blier, M.D., Ph.D., Claude de Montigny, M.D., Ph.D., and Guy Debonnel, M.D. The increase of extracellular 5-HT in brain terminal regions antagonist WAY 100635 (100 g/kg, i.v.). A short-term produced by the acute administration of 5-HT reuptake treatment with VLX (20 mg/kg/day ϫ 2 days) resulted in a inhibitors (SSRI’s) is hampered by the activation of ca. 90% suppression of the firing activity of 5-HT neurons somatodendritic 5-HT1A autoreceptors in the raphe nuclei. in the dorsal raphe nucleus. This was prevented by the The present in vivo electrophysiological studies were coadministration of (-)pindolol (15 mg/kg/day ϫ 2 days). undertaken, in the rat, to assess the effects of the Taken together, these results indicate that (-)pindolol coadministration of venlafaxine, a dual 5-HT/NE reuptake potentiated the activation of postsynaptic 5-HT1A receptors inhibitor, and (-)pindolol on pre- and postsynaptic 5-HT1A resulting from 5-HT reuptake inhibition probably by receptor function. The acute administration of venlafaxine blocking the somatodendritic 5-HT1A autoreceptor, but not and of the SSRI paroxetine (5 mg/kg, i.v.) induced a its postsynaptic congener. These results support and extend suppression of the firing activity of dorsal hippocampus CA3 previous findings providing a biological substratum for the pyramidal neurons. This effect of venlafaxine was markedly efficacy of pindolol as an accelerating strategy in major potentiated by a pretreatment with (-)pindolol (15 mg/kg, depression. -

ZHONG-THESIS-2016.Pdf
ANTIDEPRESSANTMINING AZ AMAZ DESIGNING A WEBBASED ALGORITHM AND VISUAL LANGUAGE FOR ANTIDEPRESSANT DRUG SELECTION TO EDUCATE PRIMARY CARE PRACTITIONERS By Amy Zhong A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts Baltimore, Maryland March, 2016 © 2016 Amy Zhong All Rights Reserved ABSTRACT Depression is a common mental disorder that affects approximately 14.8 million American adults each year. In addition to being a debilitating condition, depression often occurs in tandem with other medical conditions such as diabetes, heart disease, and cancer. While psychiatric professionals are essential for the management of mental health, majority of patients seek care from their primary care practitioners. This phenomenon is of great concern because diagnosis of depression within primary care settings has only been accurate 25-50% of the time. The antidepressant drug selection algorithm utilizes a unique formula to integrate patient and family medical histories, patient symptoms, and patient preferences to make optimal treatment selections. The development of a visual language explores the use of graphic elements to improve understanding of major pharmacological mechanisms, knowledge essential to making rational DQWLGHSUHVVDQWGUXJVHOHFWLRQV,QFUHDWLQJWKLVPRELOHZHEEDVHGDSSOLFDWLRQZHKRSHWR¿OOD void in resources available to primary care practitioners, and improve management of mental health within the primary care setting. By Amy Zhong Chairpersons of the Supervisory Committee Adam I. Kaplin, M.D., Ph.D., esis Preceptor Assistant Professor, Departments of Psychiatry and Neurology e Johns Hopkins University School of Medicine Kristen Rahn Hollinger, Ph.D., esis Preceptor Instructor, Departments of Psychiatry and Neurology e Johns Hopkins University School of Medicine Jennifer E. -

Strategies for Managing Sexual Dysfunction Induced by Antidepressant Medication
King’s Research Portal DOI: 10.1002/14651858.CD003382.pub3 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Taylor, M. J., Rudkin, L., Bullemor-Day, P., Lubin, J., Chukwujekwu, C., & Hawton, K. (2013). Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database of Systematic Reviews, (5). https://doi.org/10.1002/14651858.CD003382.pub3 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Summary of Drug Limitations Mary C
RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. MAYHEW SECRETARY **Medications listed in this document may or may not require a prior authorization. Please view the Preferred Drug List at: http://ahca.myflorida.com/Medicaid/Prescribed_Drug/pharm_thera/fmpdl.shtml** Summary of Drug Limitations Abilify (aripiprazole) 2mg, 5mg, 20mg, 30mg tablets Minimum age = 6; Maximum of 1 tablet per day Abilify (aripiprazole) 10mg, 15mg tablets Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17 Maximum of 1 tablet per day Abilify (aripiprazole) Discmelt 10mg, 15mg tabs Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17; Maximum of 2 tablets per day Abilify (aripiprazole) 1mg/ml solution Minimum age = 6; Maximum of 15ml per day for ages = 6 - 11; Maximum of 30ml per day for ages = 12-17; Maximum of 30ml per day for ages =/> 18 Abilify Maintena (aripiprazole) syringe/vial Minimum age = 18; Maximum of 1 syringe or vial every 28 days Absorica (isotretinoin) 10mg, 20mg, 25mg,30mg, 35mg, & Minimum age = 12 40mg capsules Abstral (fentanyl citrate) sublingual tablets Minimum age = 18; Maximum of 4 sublingual tablets per day Acanya (benzoyl peroxide/clindamycin)Gel, gel pump Minimum Age= 12 Accolate (zafirlukast) tablets Maximum of 3 tablets per day Aciphex (rabeprazole) 5mg, 10mg sprinkle capsules Minimum age = 1; Maximum age = 11; Maximum of 1 capsule per day Aciphex (rabeprazole) 20mg tablets Minimum age = 1; Maximum of 2 tablets per day Actemra (tocilizumab) 80mg/4ml, 200mg/10ml, Minimum age= 2 400mg/20ml Vials, & 162mg/0.9ml Syringe Actimmune (Interferon Gamma-1b) Maximum of 6ml every 28days Actiq (fentanyl citrate) Lozenges Minimum age = 18; Maximum of 4 lozenges per day Activella (estradiol/norethindrone) tablets Minimum age = 18 Updated 02/28/2019 1 RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. -

XERMELO™ (Telotristat Ethyl)
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 5/18/2017 SECTION: DRUGS LAST REVIEW DATE: 5/20/2021 LAST CRITERIA REVISION DATE: 5/20/2021 ARCHIVE DATE: XERMELO™ (telotristat ethyl) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. BLUE CROSS®, BLUE SHIELD® and the Cross and Shield Symbols are registered service marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans. -

M2021: Pharmacogenetic Testing
Pharmacogenetic Testing Policy Number: AHS – M2021 – Pharmacogenetic Prior Policy Name and Number, as applicable: Testing • M2021 – Cytochrome P450 Initial Presentation Date: 06/16/2021 Revision Date: N/A I. Policy Description Pharmacogenetics is defined as the study of variability in drug response due to heredity (Nebert, 1999). Cytochrome (CYP) P450 enzymes are a class of enzymes essential in the synthesis and breakdown metabolism of various molecules and chemicals. Found primarily in the liver, these enzymes are also essential for the metabolism of many medications. CYP P450 are essential to produce many biochemical building blocks, such as cholesterol, fatty acids, and bile acids. Additional cytochrome P450 are involved in the metabolism of drugs, carcinogens, and internal substances, such as toxins formed within cells. Mutations in CYP P450 genes can result in the inability to properly metabolize medications and other substances, leading to increased levels of toxic substances in the body. Approximately 58 CYP genes are in humans (Bains, 2013; Tantisira & Weiss, 2019). Thiopurine methyltransferase (TPMT) is an enzyme that methylates azathioprine, mercaptopurine and thioguanine into active thioguanine nucleotide metabolites. Azathioprine and mercaptopurine are used for treatment of nonmalignant immunologic disorders; mercaptopurine is used for treatment of lymphoid malignancies; and thioguanine is used for treatment of myeloid leukemias (Relling et al., 2011). Dihydropyrimidine dehydrogenase (DPD), encoded by the gene DPYD, is a rate-limiting enzyme responsible for fluoropyrimidine catabolism. The fluoropyrimidines (5-fluorouracil and capecitabine) are drugs used in the treatment of solid tumors, such as colorectal, breast, and aerodigestive tract tumors (Amstutz et al., 2018). A variety of cell surface proteins, such as antigen-presenting molecules and other proteins, are encoded by the human leukocyte antigen genes (HLAs). -

Flibanserin (Addyi)
STEPS New Drug Reviews Flibanserin (Addyi) for Hypoactive Sexual Desire Disorder in Premenopausal Women HARRY HOLT, MD, and JEFFREY TINGEN, PharmD, MBA, University of Virginia Health System, Charlottesville, Virginia STEPS new drug reviews Flibanserin (Addyi) is labeled for the treatment of acquired, generalized hypoactive sexual cover Safety, Tolerability, desire disorder (HSDD) in premenopausal women. It is a nonhormonal medication that Effectiveness, Price, and 1 Simplicity. Each indepen- affects serotonin receptors to increase libido. dent review is provided by authors who have no financial association with Drug Dosage Dose form Cost* the drug manufacturer. Flibanserin (Addyi) 100 mg per day at bedtime 100-mg tablet $830 This series is coordinated by Allen F. Shaughnessy, *—Estimated retail price of one month’s treatment based on information obtained at http://www.goodrx.com PharmD, MMedEd, (accessed April 1, 2016). Contributing Editor. A collection of STEPS pub- lished in AFP is available at http://www.aafp.org/ SAFETY EFFECTIVENESS afp/steps. The main risks of flibanserin are hypoten- Flibanserin has been evaluated in three ran- sion (2%) and syncope (0.4%), which are domized, double-blind, placebo-controlled more likely to occur in patients who have studies of 2,375 premenopausal women also ingested alcohol. For this reason, women with acquired, generalized HSDD, defined should not drink alcohol when taking fliban- as low sexual desire causing marked dis- serin; about one in six persons taking this tress or interpersonal difficulties.2 Women combination will experience clinically signif- in monogamous, heterosexual relationships icant hypotension and syncope.1 Flibanserin with no known cause of HSDD reported should not be taken by women with hepatic an average increase of 1.6 to 2.5 additional impairment or women who are also taking satisfying sexual events per month with treat- moderate or strong cytochrome P450 3A4 ment, from a baseline of 2.5 to 3.0 per month. -

Dosing and Monitoring: Children and Adolescents
Dosing and Monitoring: Children and Adolescents Glenn S. Hirsch, MD Keith S. Ditkowski, MD Child & Adolescent Dosing.indd 1 08-01-2018 14:56:52 Adapted from Child & Adolescent Dosing.indd 76 08-01-2018 14:56:50 DISCLAIMER This pocket reference is provided as a service to medicine by the publisher, Medworks Media Inc. This review does not imply the publisher’s agreement with the views expressed herein. Although every effort has been made to ensure that drug doses and other information are presented accurately in this publication, the ultimate responsibility rests with the prescribing physician. Neither the publisher, nor the authors can be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound currently under clinical investigation. In an effort to allow for the widest distribution of these guidelines, the authors have modified the originally printed material to more closely conform to the limitations of product labeling. For many of the drugs discussed herein, initiation at lower doses may increase tolerability and efficacy. Copyright ©2018, MedWorks Media Inc., 2205 Rockefeller Lane, Redondo Beach, CA 90278. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form. Child & Adolescent Dosing.indd 2 08-01-2018 14:56:52 Dosing and Monitoring: Children and Adolescents Glenn S. Hirsch, MD Keith S. Ditkowski, MD Dr. Hirsch is the deputy director of the New York University Child Study Center, the medical director in the Division of Child and Adolescent Psychiatry, and the assistant professor of psychiatry at the New York University School of Medicine. -

Antibiotic Practice Change to Curtail Linezolid Use in Pediatric Hospitalized Patients in Hawai‘I with Uncomplicated Skin and Soft Tissue Infections
Antibiotic Practice Change to Curtail Linezolid Use in Pediatric Hospitalized Patients in Hawai‘i with Uncomplicated Skin and Soft Tissue Infections Cheryl Okado MD and Tori Teramae BS Abstract MRSA coverage, clindamycin became a widely-used antibi- otic for treating uncomplicated SSTIs in children. Following Antimicrobial resistance affects health care providers’ choice of antibiotics increasing clindamycin use, increasing clindamycin resistance in the treatment of skin and soft tissue infections (SSTIs). Based on local was soon noted, particularly in MRSA isolates.2 Prior to 2010, antibiotic susceptibility data showing high clindamycin resistance and high MRSA predominance appeared to peak, and since then it has MRSA prevalence, a change in antibiotic regimen for children hospitalized for 3 uncomplicated SSTIs was instituted in an attempt to curb the use of linezolid. been decreasing. The prevalence of clindamycin-resistant GAS A retrospective chart review was performed on 278 pediatric patients with has been known to vary with time and location with US rates uncomplicated SSTIs hospitalized at Kapi‘olani Medical Center for Women ranging from 4%-41% since the early 2000s.4 and Children in Hawai‘i from May 2014 to April 2015 and November 2015 to October 2016. Data consisted of 12 months of baseline data and 12 In Hawai‘i, methicillin and clindamycin resistance patterns of months of data post-implementation of an antibiotic combination regimen of SA initially followed similar increasing trends. Antibiograms 2 widely-used antibiotics: high-dose cefazolin and high-dose clindamycin. at Hawai‘i’s children’s hospital, Kapi‘olani Medical Center for Practitioners were encouraged to use cefazolin alone if clinical suspicion was high for single-organism infection with group A streptococcus. -

(Flibanserin) (Flibanserin) Tablets
™ MEDICATION GUIDE addyi ADDYI™ (add-ee) (flibanserin) (flibanserin) Tablets Read this Medication Guide before you start taking ADDYI™ and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor. What is the most important information I should know about ADDYI? Your risk of severe low blood pressure and fainting (loss of consciousness) is increased if you take ADDYI and: • drink alcohol. Do not drink alcohol if you take ADDYI. • take certain prescription medicines, over-the-counter medicines, or herbal supplements. Do not take or start taking any prescription medicines, over-the-counter medicines, or herbal supplements while taking ADDYI until you have talked with your doctor. Your doctor will tell you if it is safe to take other medicines or herbal supplements while you are taking ADDYI. • have liver problems. Do not take ADDYI if you have liver problems. If you take ADDYI and you feel lightheaded or dizzy, lie down right away. Get emergency medical help or ask someone to get emergency medical help for you if the symptoms do not go away or if you faint (lose consciousness). If you faint (lose consciousness), tell your doctor as soon as you can. ADDYI is only available through the ADDYI Risk Evaluation and Mitigation Strategy (REMS) Program because of the increased risk of severe low blood pressure and fainting (loss of consciousness) with alcohol use. You can only get ADDYI from pharmacies that are enrolled in the ADDYI REMS Program. For more information about the Program and a list of pharmacies that are enrolled in the ADDYI REMS Program, go to www.AddyiREMS.com or call 1-844-PINK-PILL (1-844- 746-5745). -
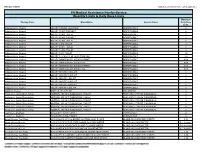
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

Drug News Nov Issue 25
D r u g N e w s 藥 物 情 報 Issue No. 25 : November 2011 This is a monthly digest of local and overseas drug safety news and information released in the previous month. For the latest news and information, please refer to public announcements or the website of the Drug Office of the Department of Health (http://www.drugoffice.gov.hk). Safety Update US: Updated information about drug Canada and FDA released similar alerts on the interaction of Zyvox (linezolid) and interaction between methylene blue and serotonin Methylene Blue with serotonergic reuptake inhibitors. At the same time, FDA also released alert on the interaction between linezolid psychiatric medications and serotonin reuptake inhibitors. The news had 20 October 2011 – The Food and Drug been reported in Issue No. 17 and 22 of Drug News Administration (FDA) provided additional respectively. In addition, the Department of Health information about the possible serotonin syndrome (DH) issued press statement on 18 February 2011 in patients taking serotonergic psychiatric and letters were also sent to healthcare professionals medications and linezolid/ methylene blue. It was in February 2011 and July 2011. found that not all serotonergic psychiatric drugs had The product certificate holders of the an equal capacity to cause serotonin syndrome with pharmaceutical products containing methylene blue, use of linezolid/ methylene blue. According to the linezolid and serotonergic psychiatric medications FDA's Adverse Event Reporting System (AERS), have been requested to update the sales pack and/or most cases of serotonin syndrome with linezolid or package insert of these products to include methylene blue occurred in patients taking specific information about the potential drug interactions.