Lepidopteran Forest Defoliators in a Changing Climate: Performance in Different Life-History Stages, and Range Expansion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forestry Department Food and Agriculture Organization of the United Nations
Forestry Department Food and Agriculture Organization of the United Nations Forest Health & Biosecurity Working Papers OVERVIEW OF FOREST PESTS ROMANIA January 2007 Forest Resources Development Service Working Paper FBS/28E Forest Management Division FAO, Rome, Italy Forestry Department DISCLAIMER The aim of this document is to give an overview of the forest pest1 situation in Romania. It is not intended to be a comprehensive review. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. © FAO 2007 1 Pest: Any species, strain or biotype of plant, animal or pathogenic agent injurious to plants or plant products (FAO, 2004). Overview of forest pests - Romania TABLE OF CONTENTS Introduction..................................................................................................................... 1 Forest pests and diseases................................................................................................. 1 Naturally regenerating forests..................................................................................... 1 Insects ..................................................................................................................... 1 Diseases................................................................................................................ -

2019 UDAF Insect Report
2019 Insect Report UTAH DEPARTMENT OF AGRICULTURE AND FOOD DIVISION OF PLANT INDUSTRY LARGE PINE WEEVIL H y l o b i u s a b i e ti s ( L i n n a e u s ) PROGRAM 2019 PARTNERS Insect Report MORMON CRICKET - VELVET LONGHORNED BEETLE - EMERALD ASH BORER - NUN MOTH - JAPANESE BEE- TLE - PINE SHOOT BEETLE - APPLE MAGGOT - GYPSY MOTH - PLUM CURCULIO - CHERRY FRUIT FLY - LARGE PINE WEEVIL - LIGHT BROWN APPLE MOTH - ROSY GYPSY MOTH - EUROPEAN HONEY BEE - BLACK FIR SAW- YER - GRASSHOPPER - MEDITERRANEAN PINE ENGRAVER - SIX-TOOTHED BARK BEETLE - NUN MOTH - EU- ROPEAN GRAPEVINE MOTH - SIBERIAN SILK MOTH - PINE TREE LAPPET - MORMON CRICKET - VELVET LONGHORNED BEETLE - EMERALD ASH BORER - NUN MOTH - JAPANESE BEETLE - PINE SHOOT BEETLE - AP- PLE MAGGOT - GYPSY MOTH - PLUM CURCULIO - CHERRY FRUIT FLY - LARGE PINE WEEVIL - LIGHT BROWN APPLE MOTH - ROSY GYPSY MOTH - EUROPEAN HONEY BEE - BLACK FIR SAWYER - GRASSHOPPER - MEDI- TERRANEAN PINE ENGRAVER - SIX-TOOTHED BARK BEETLE - NUN MOTH - EUROPEAN GRAPEVINE MOTH - SIBERIAN SILK MOTH - PINE TREE LAPPET - MORMON CRICKET - VELVET LONGHORNED BEETLE - EMERALD ASH BORER - NUN MOTH - JAPANESE BEETLE - PINE SHOOT BEETLE - APPLE MAGGOT - GYPSY MOTH - PLUM CURCULIO - CHERRY FRUIT FLY - LARGE PINE WEEVIL - LIGHT BROWN APPLE MOTH - ROSY GYPSY MOTH - EUROPEAN HONEY BEE - BLACK FIR SAWYER - GRASSHOPPER - MEDITERRANEAN PINE ENGRAVER - SIX-TOOTHED BARK BEETLE - NUN MOTH - EUROPEAN GRAPEVINE MOTH - SIBERIAN SILK MOTH - PINE TREE LAPPET - MORMON CRICKET - VELVET LONGHORNED BEETLE - EMERALD ASH BORER - NUN MOTH - JAPANESE -

Barrowhill, Otterpool and East Stour River)
Folkestone and Hythe Birds Tetrad Guide: TR13 D (Barrowhill, Otterpool and East Stour River) The tetrad TR13 D is an area of mostly farmland with several small waterways, of which the East Stour River is the most significant, and there are four small lakes (though none are publically-accessible), the most northerly of which is mostly covered with Phragmites. Other features of interest include a belt of trees running across the northern limit of Lympne Old Airfield (in the extreme south edge of the tetrad), part of Harringe Brooks Wood (which has no public access), the disused (Otterpool) quarry workings and the westernmost extent of Folkestone Racecourse and. The northern half of the tetrad is crossed by the major transport links of the M20 and the railway, whilst the old Ashford Road (A20), runs more or less diagonally across. Looking south-west towards Burnbrae from the railway Whilst there are no sites of particular ornithological significance within the area it is not without interest. A variety of farmland birds breed, including Kestrel, Stock Dove, Sky Lark, Chiffchaff, Blackcap, Lesser Whitethroat, Yellowhammer, and possibly Buzzard, Yellow Wagtail and Meadow Pipit. Two rapidly declining species, Turtle Dove and Spotted Flycatcher, also probably bred during the 2007-11 Bird Atlas. The Phragmites at the most northerly lake support breeding Reed Warbler and Reed Bunting. In winter Fieldfare and Redwing may be found in the fields, whilst the streams have attracted Little Egret, Snipe and, Grey Wagtail, with Siskin and occasionally Lesser Redpoll in the alders along the East Stour River. Corn Bunting may be present if winter stubble is left and Red Kite, Peregrine, Merlin and Waxwing have also occurred. -

Hawk-Moths, Family Sphingidae and Forewings Browner
Hawk-moths, Family Sphingidae and forewings browner. Wings normally held roof-wise along the body when at rest. Distinctive medium to large moths. Power• Larva green, striped with brown. ful fliers, generally with rather narrow, Habitat More sedentary than above pointed forewings. Most larvae are large, species, living mainly in rough flowery striped, and have a 'horn' at the tail end. places where Privet occurs. Status and distributfon Local in S Convolvulus Hawk-moth Britain, widespread on the Continent. Agrilfs c()llu()lulfli Season 6-7. A strikingly large moth; wingspan up to 12cm. Forewings greyish, marbled; hind• Poplar Hawk-moth wings browner. The abdomen is striped La()th()c l)()fJlfli with red, white and black. The proboscis A medium-sized hawk-moth; wingspan up may be up to 13cm long! to 90mm. Wings greyish to pinkish-brown, Habitat A migrant into N Europe from broadly banded, with a single white mark in the Mediterranean area, which may occur the centre of the forewings. Hindwings wherever there are flowers, especially Petu• orange-red at base, usually concealed, and nia and Nicotiana. Breeds on Convolvulus, but show in front of forewings at rest. Larvae only rarely does so in N Europe. green with yellow stripes. Status and distribution Very variable in Habitat A variety of habitats, associated numbers, regularly reaching S England, but with Sallow, Poplar and Aspen. not necessarily going further. Status and distribution Widely distrib• Season 6-9. uted and moderately common throughout the region. Death's Head Hawk-Moth Season 5-9. Achcrontia atrofJos Similar species An extraordinary insect, unlike anything Pine Hawk-moth Hyloicus pinostri is also else. -
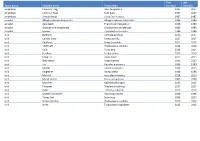
Taxon Group Common Name Taxon Name First Recorded Last
First Last Taxon group Common name Taxon name recorded recorded amphibian Common Frog Rana temporaria 1987 2017 amphibian Common Toad Bufo bufo 1987 2017 amphibian Smooth Newt Lissotriton vulgaris 1987 1987 annelid Alboglossiphonia heteroclita Alboglossiphonia heteroclita 1986 1986 annelid duck leech Theromyzon tessulatum 1986 1986 annelid Glossiphonia complanata Glossiphonia complanata 1986 1986 annelid leeches Erpobdella octoculata 1986 1986 bird Bullfinch Pyrrhula pyrrhula 2016 2017 bird Carrion Crow Corvus corone 2017 2017 bird Chaffinch Fringilla coelebs 2015 2017 bird Chiffchaff Phylloscopus collybita 2014 2016 bird Coot Fulica atra 2014 2014 bird Fieldfare Turdus pilaris 2015 2015 bird Great Tit Parus major 2015 2015 bird Grey Heron Ardea cinerea 2013 2017 bird Jay Garrulus glandarius 1999 1999 bird Kestrel Falco tinnunculus 1999 2015 bird Kingfisher Alcedo atthis 1986 1986 bird Mallard Anas platyrhynchos 2014 2015 bird Marsh Harrier Circus aeruginosus 2000 2000 bird Moorhen Gallinula chloropus 2015 2015 bird Pheasant Phasianus colchicus 2017 2017 bird Robin Erithacus rubecula 2017 2017 bird Spotted Flycatcher Muscicapa striata 1986 1986 bird Tawny Owl Strix aluco 2006 2015 bird Willow Warbler Phylloscopus trochilus 2015 2015 bird Wren Troglodytes troglodytes 2015 2015 bird Yellowhammer Emberiza citrinella 2000 2000 conifer Douglas Fir Pseudotsuga menziesii 2004 2004 conifer European Larch Larix decidua 2004 2004 conifer Lawson's Cypress Chamaecyparis lawsoniana 2004 2004 conifer Scots Pine Pinus sylvestris 1986 2004 crustacean -

پروانه ابريشم باف Tussock Moth Lymantria Monacha (Linnaeus)
وزارت ﺟﻬﺎد ﻛﺸﺎورزي ﺳﺎزﻣﺎن ﺣﻔﻆ ﻧﺒﺎﺗﺎت ﻛﺸﻮر راﻫﻨﻤﺎي ﺷﻨﺎﺳﺎﺋﻲ و ردﻳﺎﺑﻲ آﻓﺖ ﻗﺮﻧﻄﻴﻨﻪ ﺧﺎرﺟﻲ ﭘﺮواﻧﻪ اﺑﺮﻳﺸﻢ ﺑﺎف Tussock moth Lymantria monacha (Linnaeus) Lepidoptera: Lymantriidae ﺗﻬﻴﻪ و ﺗﻨﻈﻴﻢ: اﺣﻤﺪ ﭼﺮاﻏﻴﺎن دﻓﺘﺮ ﭘﺎﻳﺶ و ﺗﺤﻠﻴﻞ ﺧﻄﺮ ﺑﻬﺎر1398 ﭘﺮواﻧﻪ اﺑﺮﻳﺸﻢ ﺑﺎف Lymantria monacha (Linnaeus) Lepidoptera: Lymantriidae Common name: Nun moth, tussock moth, black arches moth, black arched tussock moth Synonyms: Psilura monacha Linnaeus, Phalaena Bombyx monacha Linnaeus, 1758 Noctua heteroclita Müller, 1764, Bombyx eremita Hübner, 1808 Bombyx nigra Freyer, 1833, Liparis monacha var. oethiops De Se.-Lon., 1857 Psilura transiens Thierry Mieg, 1886, Lymantria transiens Lambillion, 1909 Lymantria monacha flaviventer Kruilikovsky, Lymantria monacha gracilis Kruilikovsky Lymantria fasciata Hannemann, 1916, Lymantria kusnezovi Kulossow, 1928 Lymantria brunnea Stipan, 1933, Lymantria monacha chosenibia Bryk Lymantria monacha matuta Bryk, Lymantria monacha idae Bryk Lymantria monacha lateralis Bryk, Lymantria monacha eremita Lymantria monacha nigra, Liparis monacha Linnaeus Ocneria monacha Linnaeus, Phalaena monacha Linnaeus Porthetria monacha Linnaeus اﻫﻤﻴﺖ اﻗﺘﺼﺎدي: اﻳﻦ آﻓﺖ در ﺳﺎل ﻫﺎي 1863-1852 در ﻣﻨﺎﻃﻖ ﺟﻨﮕﻠﻲ روﺳﻴﻪ ﺑﺮاي اوﻟﻴﻦ ﺑـﺎر ﻃﻐﻴـﺎن ﻛـﺮد ﻛـﻪ در آن ﺳـﺎل ﻫـﺎ در ﺳﻄﺤﻲ ﺑﺎﻟﻎ403000 ﻛﻴﻠﻮﻣﺘﺮ ﻣﺮﺑﻊ ﮔﺴﺘﺮش ﻳﺎﻓﺖ(Bejer, 1988)، ﺑﻌﺪا ﺑﺼﻮرت دوره اي در ﺳﺮﺗﺎﺳﺮ اروﭘﺎ ﺣﺎﻟﺖ ﻃﻐﻴﺎﻧﻲ ﭘﻴﺪا ﻧﻤﻮد.ﺑﻴﺸﺘﺮﻳﻦ ﻣﻴﺰان ﺧﺴﺎرت آﻓﺖ ﻣﺮﺑﻮط ﺑﻪ ﺳﺎل ﻫﺎي 1984-1978 اﺳﺖ ﻛﻪ ﺑﺎﻟﻎ ﺑﺮ 2 ﻣﻴﻠﻴﻮن ﻫﻜﺘﺎرﺟﻨﮕﻞ ﻫﺎي ﺳﻮزﻧﻲ ﺑـﺮگ (ﻳــﻚ ﺳــﻮم ﺟﻨﮕــﻞ ﻫــﺎي ﻛﺸـﻮر ﻟﻬﺴــﺘﺎن ) ﺗــﺎ ﻣﺮﺣﻠــﻪ ﻟﺨــﺖ ﺷــﺪن ﻛﺎﻣـﻞ درﺧﺘــﺎن ﻣﻴﺰﺑــﺎن را -

NEWSLETTER 40 Obedient Plant, Nepeta, Pelargonium, Inula, Liatris, Veronicastrum, Eryngium, Sidalcea, Dahlia, Chicory and Especially Lavender
Botanic Garden site supports all the “Big Six” common bumblebee species and four of their cuckoo parasites [Bombus terrestris and it’s cuckoo Bombus vestalis; Bombus lucorum; Bombus hortorum; Bombus pratorum and it’s cuckoo Bombus sylvestris; Bombus lapidarius and it’s cuckoo Bombus rupestris; and Bombus pascuorum and it’s cuckoo Bombus campestris [a melanic form - all black - of this cuckoo was seen the day before]. The foraging choices of the bumblebees included Fuchsia, NEWSLETTER 40 Obedient plant, Nepeta, Pelargonium, Inula, Liatris, Veronicastrum, Eryngium, Sidalcea, Dahlia, Chicory and especially Lavender. How many bumbles does your garden support? It would be a real contribution to bumblebee conservation if all gardens provided a wide range of foraging choices for LEICESTERSHIRE any bumble visitors, from early spring, throughout the summer and into the autumn. ENTOMOLOGICAL January 2009 * In VC55, possibly indicating climate change, some bumblebees are now thought to over- SOCIETY winter as they do further south e.g.: Bombus terrestris workers seen with pollen loads, foraging Lonicera x purpusei in my garden in January 2008. VC55 Maggie Frankum, August 2008 ___________________________________________________________________________ Indoor Meetings Programme More about the Black Arches (Lymanlia monacha) All meetings are held at Holly Hayes, 216 Birstall Road, Birstall, Leicestershire LE4 4DG. Arrive at 7:00 pm for pre-meeting refreshments. Evening session starts at 7:30 pm in the lecture room. considered a more favourable locality for moth Further to Graham Finch’s article (see trapping: clearly the moths’” think “ differently. th Newsletter 39) on the distribution of this Thursday, 19 February 2009 species in Leicestershire, I have also made „Recorder friendly leafminers‟ – Anona Finch will introduce us to the leafmining Lepidoptera that are records starting with a moth trapping session, easy to identify and show us their unique habits and lifestyles. -

Lepsoc+SEL 2018 Ottawa, Canada
LepSoc+SEL 2018 Ottawa, Canada LEPSOC+SEL 2018 COMBINED ANNUAL MEETING OF THE LEPIDOPTERISTS’ SOCIETY AND SOCIETAS EUROPAEA LEPIDOPTEROLOGICA JULY 10-15, 2018 CARLETON UNIVERSITY, OTTAWA, ONTARIO, CANADA CO-CHAIRS: Vazrick Nazari, Chris Schmidt ORGANIZING COMMITTEE: Peter Hall, Don Lafontaine, Christi Jaeger MEETING WEBSITE: Ella Gilligan, Todd Gilligan PROGRAMME: Vazrick Nazari MICROLEP MEETING: David Bettman, Todd Gilligan SYMPOSIA: Erin Campbell, Federico Riva JUDGING AND DOOR PRIZES: Charlie Covell FIELD TRIP LEADERS: Chris Schmidt, Peter Hall, Rick Cavasin PRE-CONFERENCE TRIP: Maxim Larrivée MOTHING EVENTS: Jason Dombroskie, Ashley Cole-Wick CNC ACCESS: Owen Lonsdale, Michelle Locke, Jocelyn Gill REGISTRATION DESK: Sonia Gagnon, Mariah Fleck MEETING & GROUP PHOTOS: Ranger Steve Muller LOGO AND T-SHIRT DESIGN: Vazrick Nazari, Chris Schmidt VENDORS: Atelier Jean-Paquet Cover illustration: The first published image of the Canadian Swallowtail, Papilio canadensis (Rothschild & Jordan, 1906), by Philip Henry Gosse, 1840, The Canadian Naturalist: 183. 1 Programme and Abstracts LEPSOC+SEL 2018 SCHEDULE OF EVENTS JULY 10-15, 2018 Tuesday, July 10th 8:00 am — 4:30 pm Carleton University campus housing check-in Microlepidopterists' Meeting (Salon A, K.W. Neatby Building) 8:30 am — 4:30 pm moderators: Vazrick Nazari & Christi Jaeger Opening remarks A review of the tribe Cochylini (Lepidoptera, Byun Tortricidae) in Korea Collecting in rural Quebec: Highlights from the last Charpentier seasons Eiseman Leafminers of the Southwestern United States 8:30 am — 10:15 am Hayden Three gelechioid pests in Florida (In no particular order) Pyraloidea research in Nicaragua; preliminary results B. Landry based on two field trips Crasimorpha vs Oestomorpha (Gelechiidae) for the bio-control of the Brazilian Pepper Tree (Schinus J.-F. -

Lymantria Monacha
PART II Profiles of selected forest pests INSECT PESTS 103 Lymantria monacha Other scientific names: Psilura monacha Linnaeus; Liparis monacha Linnaeus; Ocneria monacha Linnaeus; Phalaena monacha Linnaeus; Porthetria monacha Linnaeus; Bombyx monacha Linnaeus, 1758; Noctua heteroclita Müller, 1764; Bombyx eremita Hübner, 1808; Bombyx nigra Freyer, 1833; Liparis monacha var. oethiops De Selys-Longchamps, 1857; Psilura transiens Thierry Mieg, 1886; Lymantria transiens Lambillion, 1909; Lymantria monacha flaviventer Kruilikovsky; Lymantria monacha gracilis Kruilikovsky; Lymantria fasciata Hannemann, 1916; Lymantria kusnezovi Kulossow, 1928; Lymantria brunnea Stipan, 1933; Lymantria monacha chosenibia Bryk; Lymantria monacha matuta Bryk; Lymantria monacha idae Bryk; Lymantria monacha lateralis Bryk; Lymantria monacha eremita; Lymantria monacha nigra Order and Family: Lepidoptera: Lymantriidae Common names: nun moth; tussock moth; black arches moth; black-arched tussock moth Lymantria monacha Linnaeus, 1758 is a major pest of broadleaved and coniferous trees in Europe and Asia. Defoliation by nun moth larvae can kill host trees especially conifers and has caused extensive losses despite intervention with biological and chemical insecticides. In parts of Europe, the occurrence of outbreaks has increased possibly as a result of the establishment of extensive pine plantations in poor quality areas. A serious outbreak was recorded in Europe between 1978 and 1985 and again in 1992 in Poland which involved hundreds of thousands of hectares. BUGWOOD.ORG/D. ADAM/2515023 BUGWOOD.ORG/H. LEMME/1260001 Lymantria monacha larva and adults (female and male) 104 Global review of forest pests and diseases DISTRIBUTION Native: Asia and the Pacific; Europe Introduced: No records to date IDENTIFICATION Adult nun moths are moderately sized, hairy and often stout-bodied. -

Lymantria (Nyctria) flavida by Paul W
he Forest Health Technology Enterprise Team (FHTET) was created in 1995 Tby the Deputy Chief for State and Private Forestry, USDA Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ Cover design by J. Marie Metz and Chuck Benedict. Photo of Lymantria (Nyctria) flavida by Paul W. Schaefer. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. The use of trade, firm, or corporation names in this publication is for information only and does not constitute an endorsement by the U.S. Department of Agriculture. Federal Recycling Program Printed on recycled paper. A REVIEW OF SELECTED SPECIES OF LYMANTRIA HÜBNER [1819] (LEPIDOPTERA: NOCTUIDAE: LYMANTRIINAE) FROM SUBTROPICAL AND TEMPERATE REGIONS OF ASIA, INCLUDING THE DESCRIPTIONS OF THREE NEW SPECIES, SOME POTENTIALLY INVASIVE TO NORTH AMERICA Michael G. -

Butterflies and Moths of Pacific Northwest Forests and Woodlands: Rare, Endangered, and Management- Sensitive Species
he Forest Health Technology Enterprise Team (FHTET) was created in 1995 by the Deputy Chief for State and Private TForestry, USDA Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ United States Depart- US Forest US Forest Service ment of Agriculture Service Forest Health Technology Enterprise Team Cover design Chuck Benedict. Photo, Taylor’s Checkerspot, Euphydryas editha taylori. Photo by Dana Ross. See page 38. For copies of this publication, contact: Dr. Jeffrey C. Miller Richard Reardon Oregon State University FHTET, USDA Forest Service Department of Rangeland Ecology 180 Canfield Street and Management Morgantown, WV 26505 202 Strand Agriculture Hall 304-285-1566 Corvallis, Washington, USA [email protected] 97331-2218 FAX 541-737-0504 Phone 541-737-5508 [email protected] The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program informa- tion (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Inde- pendence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). -

TR13 L (Hythe Ranges East and Fisherman's Beach)
Folkestone and Hythe Birds Tetrad Guide: TR13 L (Hythe Ranges East and Fisherman’s Beach) The tetrad TR13 L comprises more than 50% sea, with most of the land being part of the Hythe Ranges which is predominately open shingle with some scattered vegetation. Consequently the range of breeding species present is rather limited but probably includes Ringed Plover, Red-legged Partridge and Black Redstart and possibly Cuckoo, Oystercatcher, Nightingale, Wheatear and Stonechat. Turtle Dove bred until relatively recently and there was a Little Tern colony known at the ranges from 1909 and apparently moved progressively westward as range activity increased during the twentieth century, disappearing completely by 1968. It was the terns which attracted Roger Norman’s attention in the late 1940s and he watched the site regularly during the 1950s and 1960s, and again from 1990, though focusing mainly on the section within TR13 G in the earlier years. The bushes attract migrants in spring and autumn, with sightings having included Garden Warbler, Sedge Warbler, Redstart and Spotted Flycatcher, amongst the commoner species. The seawall and open areas can hold Wheatears and Whinchats, with hirundines, pipits, wagtails, finches and buntings passing overhead. Rarer species seen in recent years have included Wood Lark (in December 2014 and 2017), Hoopoe (in April 2015) and Dartford Warbler (in December 2017). The foreshore is worth checking for waders, gulls and terns, with regular Sanderling and Turnstone, and occasionally Grey Plover, Dunlin, Purple Sandpiper and Whimbrel, whilst Bar-tailed and Black-tailed Godwits have been noted passing offshore. Gulls may include Mediterranean Gull and Little Gull, whilst Arctic Tern, Black Tern and Little Tern have been recorded in recent years.