The Effect of Soy Isoflavones on Steroid Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2018/01/17/dmd.117.078428.DC1 1521-009X/46/4/367–379$35.00 https://doi.org/10.1124/dmd.117.078428 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:367–379, April 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans s Timothy Wong, Zhican Wang, Brian D. Chapron, Mizuki Suzuki, Katrina G. Claw, Chunying Gao, Robert S. Foti, Bhagwat Prasad, Alenka Chapron, Justina Calamia, Amarjit Chaudhry, Erin G. Schuetz, Ronald L. Horst, Qingcheng Mao, Ian H. de Boer, Timothy A. Thornton, and Kenneth E. Thummel Departments of Pharmaceutics (T.W., Z.W., B.D.C., M.S., K.G.C., C.G., B.P., Al.C., J.C., Q.M., K.E.T.), Medicine and Kidney Research Institute (I.H.d.B.), and Biostatistics (T.A.T.), University of Washington, Seattle, Washington; Department of Pharmacokinetics and Drug Metabolism, Amgen Inc., South San Francisco, California (Z.W.); Department of Pharmacokinetics and Drug Metabolism, Amgen Inc., Cambridge, Massachusetts (R.S.F.); St. Jude Children’s Research Hospital, Memphis, Tennessee Downloaded from (Am.C., E.G.S.); and Heartland Assays LLC, Ames, Iowa (R.L.H.) Received September 1, 2017; accepted January 10, 2018 ABSTRACT dmd.aspetjournals.org Metabolism of 25-hydroxyvitamin D3 (25OHD3) plays a central role in with the rates of dehydroepiandrosterone sulfonation. Further analysis regulating the biologic effects of vitamin D in the body. -

Transcriptomic Characterization of Fibrolamellar Hepatocellular
Transcriptomic characterization of fibrolamellar PNAS PLUS hepatocellular carcinoma Elana P. Simona, Catherine A. Freijeb, Benjamin A. Farbera,c, Gadi Lalazara, David G. Darcya,c, Joshua N. Honeymana,c, Rachel Chiaroni-Clarkea, Brian D. Dilld, Henrik Molinad, Umesh K. Bhanote, Michael P. La Quagliac, Brad R. Rosenbergb,f, and Sanford M. Simona,1 aLaboratory of Cellular Biophysics, The Rockefeller University, New York, NY 10065; bPresidential Fellows Laboratory, The Rockefeller University, New York, NY 10065; cDivision of Pediatric Surgery, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; dProteomics Resource Center, The Rockefeller University, New York, NY 10065; ePathology Core Facility, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; and fJohn C. Whitehead Presidential Fellows Program, The Rockefeller University, New York, NY 10065 Edited by Susan S. Taylor, University of California, San Diego, La Jolla, CA, and approved September 22, 2015 (received for review December 29, 2014) Fibrolamellar hepatocellular carcinoma (FLHCC) tumors all carry a exon of DNAJB1 and all but the first exon of PRKACA. This deletion of ∼400 kb in chromosome 19, resulting in a fusion of the produced a chimeric RNA transcript and a translated chimeric genes for the heat shock protein, DNAJ (Hsp40) homolog, subfam- protein that retains the full catalytic activity of wild-type PKA. ily B, member 1, DNAJB1, and the catalytic subunit of protein ki- This chimeric protein was found in 15 of 15 FLHCC patients nase A, PRKACA. The resulting chimeric transcript produces a (21) in the absence of any other recurrent mutations in the DNA fusion protein that retains kinase activity. -

Study Protocol and Statistical Analysis Plan
Confidential Clinical study protocol number: J1228 Page 1 Version Date: May 7, 2018 IRB study Number: NA_00067315 A Trial of maintenance Rituximab with mTor inhibition after High-dose Consolidative Therapy in CD20+, B-cell Lymphomas, Gray Zone Lymphoma, and Hodgkin’s Lymphoma Principal Investigator: Douglas E. Gladstone, MD The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins 1650 Orleans Street, CRBI-287 Baltimore, MD 21287 Phone: 410-955-8781 Fax: 410-614-1005 Email: [email protected] IRB Protocol Number: NA_00067315 Study Number: J1228 IND Number: EXEMPT Novartis Protocol Number: CRAD001NUS157T Version: May 7, 2018 Co-Investigators: Jonathan Powell 1650 Orleans Street, CRBI-443 Phone: 410-502-7887 Fax: 443-287-4653 Email: [email protected] Richard Jones 1650 Orleans Street, CRBI-244 Phone: 410-955-2006 Fax: 410-614-7279 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 2 Version Date: May 7, 2018 IRB study Number: NA_00067315 Satish Shanbhag Johns Hopkins Bayview Medical Center 301 Building, Suite 4500 4940 Eastern Ave Phone: 410-550-4061 Fax: 410-550-5445 Email: [email protected] Statisticians: Gary Rosner Phone: 410-955-4884 Email: [email protected] Marianna Zahurak Phone: 410-955-4219 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 3 Version Date: May 7, 2018 IRB study Number: NA_00067315 Table of contents Table of contents ......................................................................................................................... 3 List of abbreviations -
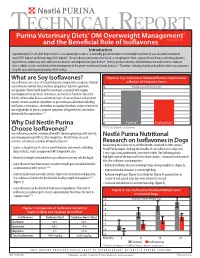
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Chronic Exposure to Bisphenol a Reduces SULT1A1 Activity in the Human Placental Cell Line Bewo
Chronic exposure to bisphenol A reduces SULT1A1 activity in the human placental cell line BeWo Pallabi Mitra Department of Pharmaceutical Chemistry University of Kansas October 27, 2006 Outline ▪ Placental structure and models ▪ Placental permeation ▪ Placental metabolism and regulation (induction/inhibition) ▪ Sulfotransferase enzymes in trophoblast ▪ Bisphenol A ▪ Effects of bisphenol A on SULT1A1 ▪ Conclusions The placental barrier The placental barrier Mother’s blood •Trophoblasts and syncytiotrophoblasts line the maternal villar surface in a monolayer- like fashion. •Constitute the rate limiting barrier to exchange between the maternal and fetal blood. Syme et al., Drug transfer and metabolism by the human placenta, Clin Pharmacokinet 2004: 43(8): 487-514 Models of the human placenta ▪ In vivo models – Anatomical and functional differences between mammalian placentas makes it difficult to extrapolate animal studies to humans. ▪ In vitro models ▪ Perfused placental cotyledon ▪ Isolated trophoblast plasma membrane ▪ Isolated transporters and receptors ▪ Villous explants ▪ Primary cultures (cytotrophoblasts) ▪ Immortalized cell lines (BeWo, JAr, JEG, HRP-1, etc.) Refn. Bode et al. In Vitro models for studying trophoblast transcellular transport, Methods Mol Med. 2006;122:225-39 Sastry, B.V., Adv Drug Deliv Rev., 1999 Jun 14. 38(1): p. 17-39. Placental permeation - Factors Efflux Carrier-mediated Passive diffusion transport Metabolism A A X A A Maternal side A X-OH A Fetal side Placental metabolism ▪ Though enzyme expression is much more restricted than hepatic metabolism, those that are functional metabolize xenobiotics as well as hormones. ▪ Placental enzymes CYP1A1/1A2, CYP19 (aromatase), GST, UGT, SULT ▪ Maternal blood-borne chemicals (drugs/polychlorinated biphenyls/pesticides) alter expression and activity. • Altered steroid metabolism. -

Regulation of Xenobiotic and Bile Acid Metabolism by the Anti-Aging Intervention Calorie Restriction in Mice
REGULATION OF XENOBIOTIC AND BILE ACID METABOLISM BY THE ANTI-AGING INTERVENTION CALORIE RESTRICTION IN MICE By Zidong Fu Submitted to the Graduate Degree Program in Pharmacology, Toxicology, and Therapeutics and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Dissertation Committee ________________________________ Chairperson: Curtis Klaassen, Ph.D. ________________________________ Udayan Apte, Ph.D. ________________________________ Wen-Xing Ding, Ph.D. ________________________________ Thomas Pazdernik, Ph.D. ________________________________ Hao Zhu, Ph.D. Date Defended: 04-11-2013 The Dissertation Committee for Zidong Fu certifies that this is the approved version of the following dissertation: REGULATION OF XENOBIOTIC AND BILE ACID METABOLISM BY THE ANTI-AGING INTERVENTION CALORIE RESTRICTION IN MICE ________________________________ Chairperson: Curtis Klaassen, Ph.D. Date approved: 04-11-2013 ii ABSTRACT Calorie restriction (CR), defined as reduced calorie intake without causing malnutrition, is the best-known intervention to increase life span and slow aging-related diseases in various species. However, current knowledge on the exact mechanisms of aging and how CR exerts its anti-aging effects is still inadequate. The detoxification theory of aging proposes that the up-regulation of xenobiotic processing genes (XPGs) involved in phase-I and phase-II xenobiotic metabolism as well as transport, which renders a wide spectrum of detoxification, is a longevity mechanism. Interestingly, bile acids (BAs), the metabolites of cholesterol, have recently been connected with longevity. Thus, this dissertation aimed to determine the regulation of xenobiotic and BA metabolism by the well-known anti-aging intervention CR. First, the mRNA expression of XPGs in liver during aging was investigated. -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

Identification of Human Sulfotransferases Involved in Lorcaserin N-Sulfamate Formation
1521-009X/44/4/570–575$25.00 http://dx.doi.org/10.1124/dmd.115.067397 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 44:570–575, April 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Identification of Human Sulfotransferases Involved in Lorcaserin N-Sulfamate Formation Abu J. M. Sadeque, Safet Palamar,1 Khawja A. Usmani, Chuan Chen, Matthew A. Cerny,2 and Weichao G. Chen3 Department of Drug Metabolism and Pharmacokinetics, Arena Pharmaceuticals, Inc., San Diego, California Received September 30, 2015; accepted January 7, 2016 ABSTRACT Lorcaserin [(R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benza- and among the SULT isoforms SULT1A1 was the most efficient. The zepine] hydrochloride hemihydrate, a selective serotonin 5-hydroxy- order of intrinsic clearance for lorcaserin N-sulfamate is SULT1A1 > Downloaded from tryptamine (5-HT) 5-HT2C receptor agonist, is approved by the U.S. SULT2A1 > SULT1A2 > SULT1E1. Inhibitory effects of lorcaserin Food and Drug Administration for chronic weight management. N-sulfamate on major human cytochrome P450 (P450) enzymes Lorcaserin is primarily cleared by metabolism, which involves were not observed or minimal. Lorcaserin N-sulfamate binds to multiple enzyme systems with various metabolic pathways in human plasma protein with high affinity (i.e., >99%). Thus, despite humans. The major circulating metabolite is lorcaserin N-sulfamate. being the major circulating metabolite, the level of free lorcaserin Both human liver and renal cytosols catalyze the formation of N-sulfamate would be minimal at a lorcaserin therapeutic dose and lorcaserin N-sulfamate, where the liver cytosol showed a higher unlikely be sufficient to cause drug-drug interactions. -

Daidzein and Genistein Content of Cereals
European Journal of Clinical Nutrition (2002) 56, 961–966 ß 2002 Nature Publishing Group All rights reserved 0954–3007/02 $25.00 www.nature.com/ejcn ORIGINAL COMMUNICATION Daidzein and genistein content of cereals J Liggins1, A Mulligan1,2, S Runswick1 and SA Bingham1,2* 1Medical Research Council Dunn Human Nutrition Unit, Hills Road, Cambridge, UK; and 2European Prospective Investigation of Cancer, University of Cambridge, Cambridge, UK Objective: To analyse 75 cereals and three soy flours commonly eaten in Europe for the phytoestrogens daidzein and genistein. Design: The phytoestrogens daidzein and genistein were extracted from dried foods, and the two isoflavones quantified after hydrolytic removal of any conjugated carbohydrate. Completeness of extraction and any procedural losses of the isoflavones 0 0 were accounted for using synthetic daidzin (7-O-glucosyl-4 -hydroxyisoflavone) and genistin (7-O-glucosyl-4 5-dihydroxyiso- flavone) as internal standards. Setting: Foods from the Cambridge UK area were purchased, prepared for eating, which included cooking if necessary, and freeze dried. Three stock soy flours were also analysed. Results: Eighteen of the foods assayed contained trace or no detectable daidzein or genistein. The soy flours were rich sources, containing 1639 – 2117 mg=kg. The concentration of the two isoflavones in the remaining foods ranged from 33 to 11 873 mg=kg. Conclusion: These analyses will supply useful information to investigators determining the intake of phytoestrogens in cereal products in order to relate intakes to potential biological activities. Sponsorship: This work was supported by the United Kingdom Medical Research Council, Ministry of Agriculture Fisheries and Food (contract FS2034) and the United States of America Army (contract DAMD 17-97-1-7028). -

Mindy Goldman, MD Clinical Professor Dept
Managing Menopause Medically and Naturally Mindy Goldman, MD Clinical Professor Dept. of Ob/Gyn and Reproductive Sciences Director, Women’s Cancer Care Program, UCSF Breast Care Center and Women’s Health University of California, San Francisco I have nothing to disclose –Mindy Goldman, MD CASE STUDY 50 yr. old G2P2 peri-menopausal woman presents with complaints of significant night sweats interfering with her ability to sleep. She has mild hot flashes during the day. She has never had a bone mineral density test but her mother had a hip fracture at age 62 due to osteoporosis. Her 46 yr. old sister was diagnosed with breast cancer at age 43, treated with lumpectomy and radiation and currently is doing well. There is no other family history of cancer. Questions 1. Would you offer her MHT? 2. If yes, how long would you continue it? 3. If no, what would you offer for alternative treatments? 4. Would your treatment differ if you knew she had underlying heart disease? Is it safe? How long can I take it? What about Mymy Bones?bones? Will it protect my heart? MHT - 2015 What about my brain? Will I get breast cancer? What about my hot flashes? Menopausal Symptoms Hot flashes Night sweats Sleep disturbances Vaginal dryness/Sexual dysfunction Mood disturbances How to Treat Menopausal Symptoms Hormone therapy Alternatives to hormones Complementary and Integrative Techniques Prior to Women’s Health Initiative Hormone therapy primary treatment of menopausal hot flashes Few women would continue hormones past one year By 1990’s well known -

Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis
separations Article Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis Feng Luan *, Li Li Tang, Xuan Xuan Chen and Hui Tao Liu College of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China; [email protected] (L.L.T.); [email protected] (X.X.C.); [email protected] (H.T.L.) * Correspondence: fl[email protected]; Tel.: +86-535-6902063 Academic Editor: Doo Soo Chung Received: 29 October 2016; Accepted: 20 December 2016; Published: 1 January 2017 Abstract: Coffee is a favorite and beverage in Western countries that is consumed daily. In the present study, capillary zone electrophoresis (CE) was applied for the separation and quantification of three isoflavones including daidzein, genistein and formononetin in coffee. Extraction of isoflavones from the coffee sample was carried out by extraction and purification process using ether after the acid hydrolysis with the antioxidant butylated hydroxy-toluene (BHT). The experimental conditions of the CE separation method were: 20 mmol/L Na2HPO4 buffer solution, 25 kV applied voltage, 3 s hydrodynamic injection at 30 mbar, and UV detection at 254 nm. The results show that the three compounds can be tested within 10 min with a linearity of 0.5–50 µg/mL for all three compounds. The limits of detection were 0.0642, 0.134, and 0.0825 µg/mL for daidzein, formononetin and genistein, respectively. The corresponding average recovery was 99.39% (Relative Standard Detection (RSD) = 1.76%), 98.71% (RSD = 2.11%) and 97.37% (RSD = 3.74%). Keywords: capillary zone electrophoresis (CE); daidzein; genistein; formononetin; acid hydrolysis 1. -

Contemporary Approaches for Managing Menopause Symptoms
Menopause 2017 Guidelines: Looking at Special Populations and Building on Existing Practice Patricia Geraghty, MSN, FNP-BC, WHNP Disclosures • Speaker Bureau • AbbVie • Therapeutics MD • Advisory Board • AbbVie (endometriosis) • Sharecare Inc. • Procedure Proctor • Bayer (Contraception) • Off label discussion will be included and identified in this discussion. Objectives • Identify the benefits and risks of estrogen, progesterone, and non-hormonal pharmacological management of menopause symptoms based on dosage, route of administration, pharmacokinetics, treatment population and duration of use. • Develop treatment strategies for special populations including premature ovarian insufficiency, prolonged symptoms, and women who are not candidates for estrogen. • Incorporate the NAMS 2017 Position Statement into patient counseling on the timeline of menopause symptoms and the termination of hormone therapy. Lifespan Lifespan Median 34 322 BC Aristotle describes transition Ages the Through Menopause yr JAMA. JAMA. Investigators. Initiative Health the Women's for Group Writing 2002;288(3):321 - 333. doi:10.1001/jama.288.3.321 Treated with plants s/a 1800’s cohosh, cannabis, opium 1821 French physician DeGardanne 53 lifespan lifespan calls it “Menopause” Adult - 1900’s Deficiency disease 55 1942 Premarin” copyright yr 1960’s “Forever Feminine” by Robert Wilson MD 1980’s-1990’s “Politics of Menopause” by Frances McCrea 2002 WHI- First large randomized control trial “My periods are different. Is This Menopause?” • Random cycling, sometimes early, sometimes late. • Flow variable with prodromal spotting, a long taper, or stopping and starting. • She has a day of very heavy flow. • 77% have duration 10+ days, heavy bleeding 3+ days, spotting 6+ days Paramsothy P, et al. BJOG. 2014 Nov;121(12):1564-73.