Calculation and Prediction of Fugacity and Activity Coefficients in Binary Mixtures, Using a Self-Regulated Fretwidth Harmony
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
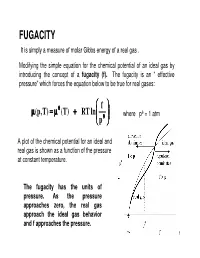
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Dissociation Constants and Ph-Titration Curves at Constant Ionic Strength from Electrometric Titrations in Cells Without Liquid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1537 Part of Journal of Research of the N.ational Bureau of Standards, Volume 30, May 1943 DISSOCIATION CONSTANTS AND pH-TITRATION CURVES AT CONSTANT IONIC STRENGTH FROM ELECTRO METRIC TITRATIONS IN CELLS WITHOUT LIQUID JUNCTION : TITRATIONS OF FORMIC ACID AND ACETIC ACID By Roger G. Bates, Gerda L. Siegel, and S. F. Acree ABSTRACT An improved method for obtaining the titration curves of monobasic acids is outlined. The sample, 0.005 mole of the sodium salt of the weak acid, is dissolver! in 100 ml of a 0.05-m solution of sodium chloride and titrated electrometrically with an acid-salt mixture in a hydrogen-silver-chloride cell without liquid junction. The acid-salt mixture has the composition: nitric acid, 0.1 m; pot assium nitrate, 0.05 m; sodium chloride, 0.05 m. The titration therefore is performed in a. medium of constant chloride concentration and of practically unchanging ionic strength (1'=0.1) . The calculations of pH values and of dissociation constants from the emf values are outlined. The tit ration curves and dissociation constants of formic acid and of acetic acid at 25 0 C were obtained by this method. The pK values (negative logarithms of the dissociation constants) were found to be 3.742 and 4. 754, respectively. CONTENTS Page I . Tntroduction __ _____ ~ __ _______ . ______ __ ______ ____ ________________ 347 II. Discussion of the titrat ion metbod __ __ ___ ______ _______ ______ ______ _ 348 1. Ti t;at~on. clU,:es at constant ionic strength from cells without ltqUld JunctlOlL - - - _ - __ _ - __ __ ____ ____ _____ __ _____ ____ __ _ 349 2. -

Molecular Thermodynamics
Chapter 1. The Phase-Equilibrium Problem Homogeneous phase: a region where the intensive properties are everywhere the same. Intensive property: a property that is independent of the size temperature, pressure, and composition, density(?) Gibbs phase rule ( no reaction) Number of independent intensive properties = Number of components – Number of phases + 2 e.g. for a two-component, two-phase system No. of intensive properties = 2 1.2 Application of Thermodynamics to Phase-Equilibrium Problem Chemical potential : Gibbs (1875) At equilibrium the chemical potential of each component must be the same in every phase. i i ? Fugacity, Activity: more convenient auxiliary functions 0 i yi P i xi fi fugacity coefficient activity coefficient fugacity at the standard state For ideal gas mixture, i 1 0 For ideal liquid mixture at low pressures, i 1, fi Psat In the general case Chapter 2. Classical Thermodynamics of Phase Equilibria For simplicity, we exclude surface effect, acceleration, gravitational or electromagnetic field, and chemical and nuclear reactions. 2.1 Homogeneous Closed Systems A closed system is one that does not exchange matter, but it may exchange energy. A combined statement of the first and second laws of thermodynamics dU TBdS – PEdV (2-2) U, S, V are state functions (whose value is independent of the previous history of the system). TB temperature of thermal bath, PE external pressure Equality holds for reversible process with TB = T, PE =P dU = TdS –PdV (2-3) and TdS = Qrev, PdV = Wrev U(S,V) is state function. The group of U, S, V is a fundamental group. Integrating over a reversible path, U is independent of the path of integration, and also independent of whether the system is maintained in a state of internal equilibrium or not during the actual process. -

Equation of State. Fugacities of Gaseous Solutions
ON THE THERMODYNAMICS OF SOLUTIONS. V k~ EQUATIONOF STATE. FUGACITIESOF GASEOUSSOLUTIONS' OTTO REDLICH AND J. N. S. KWONG Shell Development Company, Emeryville, California Received October 15, 1948 The calculation of fugacities from P-V-T data necessarily involves a differentia- tion with respect to the mole fraction. The fugacity rule of Lewis, which in effect would eliminate this differentiation, does not furnish a sufficient approximation. Algebraic representation of P-V-T data is desirable in view of the difficulty of nu- merical or graphical differentiation. An equation of state containing two individ- ual coefficients is proposed which furnishes satisfactory results above the critical temperature for any pressure. The dependence of the coefficients on the composi- tion of the gas is discussed. Relations and methods for the calculation of fugacities are derived which make full use of whatever data may be available. Abbreviated methods for moderate pressure are discussed. The old problem of the equation of state has a practical aspect which has become increasingly important in recent times. The systematic description of gas reactions under high pressure requires information about the fugacities, derived sometimes from extensive data but frequently from nothing more than t,he critical pressure and temperature. For practical purposes a representation of the relation between pressure, vol- ume, and temperature based on two or three individual coefficients is desirable, although a representation of this type satisfies the theorem of corresponding states and therefore cannot be accurate. Since the calculation of fugacities involves a differentiation with respect to the mole fraction, an algebraic repre- sentation by means of an equation of state appears to be desirable. -

Simple Mixtures
Simple mixtures Before dealing with chemical reactions, here we consider mixtures of substances that do not react together. At this stage we deal mainly with binary mixtures (mixtures of two components, A and B). We therefore often be able to simplify equations using the relation xA + xB = 1. A restriction in this chapter – we consider mainly non-electrolyte solutions – the solute is not present as ions. The thermodynamic description of mixtures We already considered the partial pressure – the contribution of one component to the total pressure, when we were dealing with the properties of gas mixtures. Here we introduce other analogous partial properties. Partial molar quantities: describe the contribution (per mole) that a substance makes to an overall property of mixture. The partial molar volume, VJ – the contribution J makes to the total volume of a mixture. Although 1 mol of a substance has a characteristic volume when it is pure, 1 mol of a substance can make different contributions to the total volume of a mixture because molecules pack together in different ways in the pure substances and in mixtures. Imagine a huge volume of pure water. When a further 1 3 mol H2O is added, the volume increases by 18 cm . When we add 1 mol H2O to a huge volume of pure ethanol, the volume increases by only 14 cm3. 18 cm3 – the volume occupied per mole of water molecules in pure water. 14 cm3 – the volume occupied per mole of water molecules in virtually pure ethanol. The partial molar volume of water in pure water is 18 cm3 and the partial molar volume of water in pure ethanol is 14 cm3 – there is so much ethanol present that each H2O molecule is surrounded by ethanol molecules and the packing of the molecules results in the water molecules occupying only 14 cm3. -

Fugacity of Condensed Media
Fugacity of condensed media Leonid Makarov and Peter Komarov St. Petersburg State University of Telecommunications, Bolshevikov 22, 193382, St. Petersburg, Russia ABSTRACT Appearance of physical properties of objects is a basis for their detection in a media. Fugacity is a physical property of objects. Definition of estimations fugacity for different objects can be executed on model which principle of construction is considered in this paper. The model developed by us well-formed with classical definition fugacity and open up possibilities for calculation of physical parameter «fugacity» for elementary and compound substances. Keywords: fugacity, phase changes, thermodynamic model, stability, entropy. 1. INTRODUCTION The reality of world around is constructed of a plenty of the objects possessing physical properties. Du- ration of objects existence – their life time, is defined by individual physical and chemical parameters, and also states of a medium. In the most general case any object, under certain states, can be in one of three ag- gregative states - solid, liquid or gaseous. Conversion of object substance from one state in another refers to as phase change. Evaporation and sublimation of substance are examples of phase changes. Studying of aggregative states of substance can be carried out from a position of physics or on the basis of mathematical models. The physics of the condensed mediums has many practical problems which deci- sion helps to study complex processes of formation and destruction of medium objects. Natural process of object destruction can be characterized in parameter volatility – fugacity. For the first time fugacity as the settlement size used for an estimation of real gas properties, by means of the thermodynamic parities de- duced for ideal gases, was offered by Lewis in 1901. -
![Lecture 4. Thermodynamics [Ch. 2]](https://docslib.b-cdn.net/cover/7153/lecture-4-thermodynamics-ch-2-1457153.webp)
Lecture 4. Thermodynamics [Ch. 2]
Lecture 4. Thermodynamics [Ch. 2] • Energy, Entropy, and Availability Balances • Phase Equilibria - Fugacities and activity coefficients -K-values • Nonideal Thermodynamic Property Models - P-v-T equation-of-state models - Activity coefficient models • Selecting an Appropriate Model Thermodynamic Properties • Importance of thermodyyppnamic properties and equations in separation operations – Energy requirements (heat and work) – Phase equilibria : Separation limit – Equipment sizing • Property estimation – Specific volume, enthalpy, entropy, availability, fugacity, activity, etc. – Used for design calculations . Separator size and layout . AiliAuxiliary components : PiPipi ing, pumps, va lves, etc. EnergyEnergy,, Entropy and Availability Balances Heat transfer in and out Q ,T Q ,T in s out s OfdOne or more feed streams flowing into the system are … … separated into two or more prodttduct streams thtflthat flow Streams in out of the system. n,zi ,T,P,h,s,b,v : Separation : : : process n Molar flow rate : (system) : z Mole fraction Sirr , LW Streams out T Temperature n,zi ,T,P,h,s,b,v P Pressure h Molar enthalpy … … (Surroundings) s Molar entropy T b Molar availability 0 (W ) (W ) s in s out v Specific volume Shaft work in and out Energy Balance • Continuous and steady-state flow system • Kinetic, potential, and surface energy changes are neglected • First law of thermodynamics (conservation of energy) (stream enthalpy flows + heat transfer + shaft work)leaving system - (t(stream en thlthalpy flows +h+ hea ttt trans fer + s hfthaft wor k)entering -

Chapter 9 Ideal and Real Solutions
2/26/2016 CHAPTER 9 IDEAL AND REAL SOLUTIONS • Raoult’s law: ideal solution • Henry’s law: real solution • Activity: correlation with chemical potential and chemical equilibrium Ideal Solution • Raoult’s law: The partial pressure (Pi) of each component in a solution is directly proportional to the vapor pressure of the corresponding pure substance (Pi*) and that the proportionality constant is the mole fraction (xi) of the component in the liquid • Ideal solution • any liquid that obeys Raoult’s law • In a binary liquid, A-A, A-B, and B-B interactions are equally strong 1 2/26/2016 Chemical Potential of a Component in the Gas and Solution Phases • If the liquid and vapor phases of a solution are in equilibrium • For a pure liquid, Ideal Solution • ∆ ∑ Similar to ideal gas mixing • ∆ ∑ 2 2/26/2016 Example 9.2 • An ideal solution is made from 5 mole of benzene and 3.25 mole of toluene. (a) Calculate Gmixing and Smixing at 298 K and 1 bar. (b) Is mixing a spontaneous process? ∆ ∆ Ideal Solution Model for Binary Solutions • Both components obey Rault’s law • Mole fractions in the vapor phase (yi) Benzene + DCE 3 2/26/2016 Ideal Solution Mole fraction in the vapor phase Variation of Total Pressure with x and y 4 2/26/2016 Average Composition (z) • , , , , ,, • In the liquid phase, • In the vapor phase, za x b yb x c yc Phase Rule • In a binary solution, F = C – p + 2 = 4 – p, as C = 2 5 2/26/2016 Example 9.3 • An ideal solution of 5 mole of benzene and 3.25 mole of toluene is placed in a piston and cylinder assembly. -

Activity and Fugacity Ray Fu
Activity and Fugacity Ray Fu Notation symbol description m, M intensive (molar) and extensive property of pure species mi, Mi as above, but specifically for species i m◦, mig, mid reference state, ideal gas, and ideal solution conditions msat saturation (liquid-vapor equilibrium) conditions mα, mβ, ml, mv α, β arbitrary and l, v liquid and vapor phases ^ f, ', fi,' ^i fugacity (coeffcient) of pure species and of species i in mixture xi, yi liquid and gas phase mole fraction of species i PT total pressure of mixture * a harder exercise Fugacity in the gas phase For an ideal gas, and assuming constant T and N, we have P dµ = dg = v dP () µ − µ◦ = g − g◦ = RT ln : (1) P ◦ Exercise 1 Prove the above identity. For non-ideal gases and fluids, we can extend this identity by defining an effective pressure f such that f f µ − µig =: RT ln = RT ln : (2) P ig f ig We call f the fugacity; it is the pressure that an ideal gas with the same chemical potential would have. By construction, f ig = P ig, and the second equality follows. The pressure P ig is the pressure of the (real) gas; the superscript ig reminds us that we are considering an ideal gas reference state. It is also convenient to define the fugacity coefficient ', satisfying 'P ig f =: 'P ig () µ − µig = RT ln = RT ln ': (3) P ig The fugacity coefficient ' contains no new thermodynamics. It simply hides all the complicated intermolec- ular interactions that real gases possess, and we can interpret it as a measure of a gas's deviation from ideality: the further the fugacity coefficient of a gas diverges from one, the less ideal is the gas. -

THE PENG-ROBINSON EQUATION of STATE Using Mathematica
$5::j class and home problems ) The object of this column is to enhance our readers' collections of interesting and novel prob lems in chemical engineering. Problems of the type that can be used to motivate the student by presenting a particular principle in class, or in a new light, or that can be assigned as a novel home problem, are requested, as well as those that are more traditional in nature and that elucidate dif ficult concepts. Manuscripts should not exceed 14 double-spaced pages and should be accompanied by the originals of any figures or photographs. Please submit them to Professor James 0. Wilkes (e-mail: [email protected]), Chemical Engineering Department, University of Michigan, Ann Arbor, Ml 48109-2136. Applications of THE PENG-ROBINSON EQUATION OF STATE Using Mathematica HousAM Brnous National Institute ofApplied Sciences and Technology • Tunis, Tunisia onsider a single component characterized by its critical where pressure, temperature, acentric factor, Pc Tc, and w. The CPeng-Robinson equation of state[24J is given by: (7) P=~- a (1) (V-b) [v(v+b)+b(V-b)] (8) where (9) i=l i=l b = 0.07780 R Tc (2) P, p pc Ai= 0.45724ai T; and B = 0 07780_l.. (10) I • T (RT )2 2 ri ri (3) a= 0.45724----t"-[ 1 +m( I-Jr:)] For each component, we define the reduced pressure and C temperature using P,, = P / Pc, and T,, = T / Tc, , and ai is (4) identified by an equation similar to Eq. (3) - given for the pure component case. The binary interaction parameter, kii , and 2 m = 0.37464 + 1.54226w-0.26992w . -

Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium
Subject Chemistry Paper No and Title 10, Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No and 11, Fugacity and Third law of thermodynamics Title Module Tag CHE_P10_M11 CHEMISTRY Paper No. 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No. 11: Fugacity and Third law of thermodynamics TABLE OF CONTENTS 1. Learning Outcomes 2. Fugacity 3. Fugacity coefficient 4. Determination of fugacity of gas 5. Physical significance of fugacity 6. Third law of thermodynamics 7. Determination of entropy for solids, liquids and gases 8. Residual entropy 9. 9.Summary CHEMISTRY Paper No. 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) Module No. 11: Fugacity and Third law of thermodynamics 1. Learning Outcomes After studying this module you shall be able to: Know the concept of fugacity Determine the coefficient of fugacity Learn about the third law of thermodynamics Know the concept of residual entropy 2. Fugacity The concept of fugacity was introduced by an American chemist, G.N Lewis. It is used for representing the real behavior of Vander Waal’s gases which is different from behavior of ideal gases. At constant temperature, the variation of free energy with pressure is given by: (/)GPV …(1) T For one mole of an ideal gas, V stands for the molar volume and thus we can write it as: ()/dG RT dP P …(2) T Similarly for n number of moles of gas, the equation turns into: (dG ) nRT dP / P nRT ln P …(3) T Integrating the equation (3) G G* nRT ln P …(4) in which G* (the integration constant) is the free energy of n moles of the gas when pressure P is unity.