Simulation Study on the Influence of Gas Mole Fraction and Aqueous
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
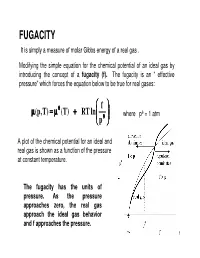
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Evaluation of UNIFAC Group Interaction Parameters Usijng Properties Based on Quantum Mechanical Calculations Hansan Kim New Jersey Institute of Technology
New Jersey Institute of Technology Digital Commons @ NJIT Theses Theses and Dissertations Spring 2005 Evaluation of UNIFAC group interaction parameters usijng properties based on quantum mechanical calculations Hansan Kim New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/theses Part of the Chemical Engineering Commons Recommended Citation Kim, Hansan, "Evaluation of UNIFAC group interaction parameters usijng properties based on quantum mechanical calculations" (2005). Theses. 479. https://digitalcommons.njit.edu/theses/479 This Thesis is brought to you for free and open access by the Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Theses by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying -

Prediction of Solubility of Amino Acids Based on Cosmo Calculaition
PREDICTION OF SOLUBILITY OF AMINO ACIDS BASED ON COSMO CALCULAITION by Kaiyu Li A dissertation submitted to Johns Hopkins University in conformity with the requirement for the degree of Master of Science in Engineering Baltimore, Maryland October 2019 Abstract In order to maximize the concentration of amino acids in the culture, we need to obtain solubility of amino acid as a function of concentration of other components in the solution. This function can be obtained by calculating the activity coefficient along with solubility model. The activity coefficient of the amino acid can be calculated by UNIFAC. Due to the wide range of applications of UNIFAC, the prediction of the activity coefficient of amino acids is not very accurate. So we want to fit the parameters specific to amino acids based on the UNIFAC framework and existing solubility data. Due to the lack of solubility of amino acids in the multi-system, some interaction parameters are not available. COSMO is a widely used way to describe pairwise interactions in the solutions in the chemical industry. After suitable assumptions COSMO can calculate the pairwise interactions in the solutions, and largely reduce the complexion of quantum chemical calculation. In this paper, a method combining quantum chemistry and COSMO calculation is designed to accurately predict the solubility of amino acids in multi-component solutions in the ii absence of parameters, as a supplement to experimental data. Primary Reader and Advisor: Marc D. Donohue Secondary Reader: Gregory Aranovich iii Contents -

Molecular Thermodynamics
Chapter 1. The Phase-Equilibrium Problem Homogeneous phase: a region where the intensive properties are everywhere the same. Intensive property: a property that is independent of the size temperature, pressure, and composition, density(?) Gibbs phase rule ( no reaction) Number of independent intensive properties = Number of components – Number of phases + 2 e.g. for a two-component, two-phase system No. of intensive properties = 2 1.2 Application of Thermodynamics to Phase-Equilibrium Problem Chemical potential : Gibbs (1875) At equilibrium the chemical potential of each component must be the same in every phase. i i ? Fugacity, Activity: more convenient auxiliary functions 0 i yi P i xi fi fugacity coefficient activity coefficient fugacity at the standard state For ideal gas mixture, i 1 0 For ideal liquid mixture at low pressures, i 1, fi Psat In the general case Chapter 2. Classical Thermodynamics of Phase Equilibria For simplicity, we exclude surface effect, acceleration, gravitational or electromagnetic field, and chemical and nuclear reactions. 2.1 Homogeneous Closed Systems A closed system is one that does not exchange matter, but it may exchange energy. A combined statement of the first and second laws of thermodynamics dU TBdS – PEdV (2-2) U, S, V are state functions (whose value is independent of the previous history of the system). TB temperature of thermal bath, PE external pressure Equality holds for reversible process with TB = T, PE =P dU = TdS –PdV (2-3) and TdS = Qrev, PdV = Wrev U(S,V) is state function. The group of U, S, V is a fundamental group. Integrating over a reversible path, U is independent of the path of integration, and also independent of whether the system is maintained in a state of internal equilibrium or not during the actual process. -

Equation of State. Fugacities of Gaseous Solutions
ON THE THERMODYNAMICS OF SOLUTIONS. V k~ EQUATIONOF STATE. FUGACITIESOF GASEOUSSOLUTIONS' OTTO REDLICH AND J. N. S. KWONG Shell Development Company, Emeryville, California Received October 15, 1948 The calculation of fugacities from P-V-T data necessarily involves a differentia- tion with respect to the mole fraction. The fugacity rule of Lewis, which in effect would eliminate this differentiation, does not furnish a sufficient approximation. Algebraic representation of P-V-T data is desirable in view of the difficulty of nu- merical or graphical differentiation. An equation of state containing two individ- ual coefficients is proposed which furnishes satisfactory results above the critical temperature for any pressure. The dependence of the coefficients on the composi- tion of the gas is discussed. Relations and methods for the calculation of fugacities are derived which make full use of whatever data may be available. Abbreviated methods for moderate pressure are discussed. The old problem of the equation of state has a practical aspect which has become increasingly important in recent times. The systematic description of gas reactions under high pressure requires information about the fugacities, derived sometimes from extensive data but frequently from nothing more than t,he critical pressure and temperature. For practical purposes a representation of the relation between pressure, vol- ume, and temperature based on two or three individual coefficients is desirable, although a representation of this type satisfies the theorem of corresponding states and therefore cannot be accurate. Since the calculation of fugacities involves a differentiation with respect to the mole fraction, an algebraic repre- sentation by means of an equation of state appears to be desirable. -

Modeling of Activity Coefficients Using Computational
Modelling of activity coefficents by comp. chem. 1 Modelling of activity coefficients using computational chemistry Eirik Falck da Silva Report in DIK 2099 Faselikevekter June 2002 Modelling of activity coefficents by comp. chem. 2 SUMMARY ......................................................................................................3 INTRODUCTION .............................................................................................3 background .............................................................................................................................................3 The use of computational chemistry .....................................................................................................4 REVIEW...........................................................................................................4 Introduction ............................................................................................................................................4 Free energy of solvation .........................................................................................................................5 Cosmo-RS ............................................................................................................................................7 SMx models..........................................................................................................................................7 Application of infinite dilution solvation energy..................................................................................8 -

Fugacity of Condensed Media
Fugacity of condensed media Leonid Makarov and Peter Komarov St. Petersburg State University of Telecommunications, Bolshevikov 22, 193382, St. Petersburg, Russia ABSTRACT Appearance of physical properties of objects is a basis for their detection in a media. Fugacity is a physical property of objects. Definition of estimations fugacity for different objects can be executed on model which principle of construction is considered in this paper. The model developed by us well-formed with classical definition fugacity and open up possibilities for calculation of physical parameter «fugacity» for elementary and compound substances. Keywords: fugacity, phase changes, thermodynamic model, stability, entropy. 1. INTRODUCTION The reality of world around is constructed of a plenty of the objects possessing physical properties. Du- ration of objects existence – their life time, is defined by individual physical and chemical parameters, and also states of a medium. In the most general case any object, under certain states, can be in one of three ag- gregative states - solid, liquid or gaseous. Conversion of object substance from one state in another refers to as phase change. Evaporation and sublimation of substance are examples of phase changes. Studying of aggregative states of substance can be carried out from a position of physics or on the basis of mathematical models. The physics of the condensed mediums has many practical problems which deci- sion helps to study complex processes of formation and destruction of medium objects. Natural process of object destruction can be characterized in parameter volatility – fugacity. For the first time fugacity as the settlement size used for an estimation of real gas properties, by means of the thermodynamic parities de- duced for ideal gases, was offered by Lewis in 1901. -

Dortmund Data Bank Retrieval, Display, Plot, and Calculation
Dortmund Data Bank Retrieval, Display, Plot, and Calculation Tutorial and Documentation DDBSP – Dortmund Data Bank Software Package DDBST - Dortmund Data Bank Software & Separation Technology GmbH Marie-Curie-Straße 10 D-26129 Oldenburg Tel.: +49 441 361819 0 [email protected] www.ddbst.com DDBSP – Dortmund Data Bank Software Package 2019 Content 1 Introduction.....................................................................................................................................................................6 1.1 The XDDB (Extended Data)..................................................................................................................................7 2 Starting the Dortmund Data Bank Retrieval Program....................................................................................................8 3 Searching.......................................................................................................................................................................10 3.1 Building a Simple Systems Query........................................................................................................................10 3.2 Building a Query with Component Lists..............................................................................................................11 3.3 Examining Further Query List Functionality.......................................................................................................11 3.4 Import Aspen Components...................................................................................................................................14 -
![Lecture 4. Thermodynamics [Ch. 2]](https://docslib.b-cdn.net/cover/7153/lecture-4-thermodynamics-ch-2-1457153.webp)
Lecture 4. Thermodynamics [Ch. 2]
Lecture 4. Thermodynamics [Ch. 2] • Energy, Entropy, and Availability Balances • Phase Equilibria - Fugacities and activity coefficients -K-values • Nonideal Thermodynamic Property Models - P-v-T equation-of-state models - Activity coefficient models • Selecting an Appropriate Model Thermodynamic Properties • Importance of thermodyyppnamic properties and equations in separation operations – Energy requirements (heat and work) – Phase equilibria : Separation limit – Equipment sizing • Property estimation – Specific volume, enthalpy, entropy, availability, fugacity, activity, etc. – Used for design calculations . Separator size and layout . AiliAuxiliary components : PiPipi ing, pumps, va lves, etc. EnergyEnergy,, Entropy and Availability Balances Heat transfer in and out Q ,T Q ,T in s out s OfdOne or more feed streams flowing into the system are … … separated into two or more prodttduct streams thtflthat flow Streams in out of the system. n,zi ,T,P,h,s,b,v : Separation : : : process n Molar flow rate : (system) : z Mole fraction Sirr , LW Streams out T Temperature n,zi ,T,P,h,s,b,v P Pressure h Molar enthalpy … … (Surroundings) s Molar entropy T b Molar availability 0 (W ) (W ) s in s out v Specific volume Shaft work in and out Energy Balance • Continuous and steady-state flow system • Kinetic, potential, and surface energy changes are neglected • First law of thermodynamics (conservation of energy) (stream enthalpy flows + heat transfer + shaft work)leaving system - (t(stream en thlthalpy flows +h+ hea ttt trans fer + s hfthaft wor k)entering -

Introduction of Cosmotherm and Its Ability to Predict Infinite Dilution
This is a postprint of Industrial & engineering chemistry research, 42(15), 3635-3641. The original version of the paper can be found under http://pubs.acs.org/doi/abs/10.1021/ie020974v Prediction of Infinite Dilution Activity Coefficients using COSMO-RS R. Putnam1, R. Taylor1,2, A. Klamt3, F. Eckert3, and M. Schiller4 1Department of Chemical Engineering, Clarkson University, Potsdam, NY 13699-5705, USA 2Department of Chemical Engineering, Universiteit Twente, Postbus 217 7500 AE Enschede The Netherlands 3COSMOlogic GmbH&Co.KG Burscheider Str. 515 51381 Leverkusen Germany 4 E.I. du Pont de Nemours and Company DuPont Engineering Technology 1007 Market Street Wilmington, DE 19808, USA Abstract Infinite dilution activity coefficients (IDACs) are important characteristics of mixtures because of their ability to predict operating behavior in distillation processes. Thermodynamic models are used to predict IDACs since experimental data can be difficult and costly to obtain. The models most often employed for predictive purposes are the Original and Modified UNIFAC Group Contribution Methods (GCMs). COSMO-RS (COnductor-like Screening MOdel for Real Solvents) is an alternative predictive method for a wide variety of systems that requires a limited minimum number of input parameters. A significant difference between GCMs and COSMO- RS is that a given GCMs’ predictive ability is dependent on the availability of group interaction parameters, whereas COSMO-RS is only limited by the availability of individual component parameters. In this study COSMO-RS was used to predict infinite dilution activity coefficients. The database assembled by, and calculations with various UNIFAC models carried out by Voutsas et al. (1996) were used as the basis for this comparison. -

Evaluation of UNIFAC Group Interaction Parameters Usijng
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT EVALUATION OF UNIFAC GROUP INTERACTION PARAMETERS USING PROPERTIES BASED ON QUANTUM MECHANICAL CALCULATIONS by Hansan Kim Crrnt rp-ntrbtn thd h ASOG nd UIAC r dl d fr pprxt ttn f xtr bhvr bt nbl t dtnh btn r At n Mll (AIM thr n lv th