Flaveria Pringlei (C3) Andflaveria Trinervia (C4) Under Nacl Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flaveria' Received for Publication February 21, 1986 R
Plant Physiol. (1986) 82, 211-217 0032-0889/86/82/0211/07/$0 1.00/0 Photosynthesis of F1 Hybrids between C4 and C3-C4 Species of Flaveria' Received for publication February 21, 1986 R. HAROLD BROWN, CAROLE L. BASSETT, RANDALL G. CAMERON, PHILIP T. EVANS, JOSEPH H. BOUTON*, CLANTON C. BLACK, JR., LEONEL O'REILLY STERNBERG, AND MICHAEL J. DENIRO Department ofAgronomy (R.H.B., P.T.E., J.H.B., R.G.C.) and Department ofBiochemistry (C.C.B.), University ofGeorgia, Athens, Georgia 30602; Richard Russell Research Center, United States Department ofAgriculture, Agricultural Research Service, Athens, Georgia (C.L.B.); Department of Biology, University ofMiami, Coral Gables, Florida 33124 (L.O'R.S.); and Department ofEarth and Space Sciences and Archaeology Program, University ofCalifornia, Los Angeles, California 90024 (M.J.D.) ABSTRACT not been found which are closely enough related to produce highly fertile offspring. But species have been discovered in Photosynthetic characteristics were studied in several F, hybrids be- several genera which have photosynthetic and leaf anatomical tween C4 and C3-C4 species of Flaveria. Stable carbon isotope ratios, 02 characteristics intermediate between C3 and C4 species (C3-C4) inhibition of apparent photosynthesis, and phosphoenolpyruvate carbox- (11, 20, 22). These intermediate characteristics may indicate ylase activities in the hybrids were similar to the means for the parents. closer phylogenetic relationships with C4 species and greater Values of CO2 compensation concentrations were nearer to those of the success in hybridization. Because C3-C4 species in most cases fix C4 parent and apparent photosynthesis was below that of both parents, CO2 exclusively by the C3 cycle (10, 11, 20, 29), hybrids between being only 60 and 74% of that of the lowest (C3-C4) parent in two C4 and C3-C4 species may be useful in understanding genetic experiments. -

Flórula Vascular De La Sierra De Catorce Y Territorios Adyacentes, San Luis Potosi, México
Acta Botanica Mexicana 78: 1-38 (2007) FLÓRULA VASCULAR DE LA SIERRA DE CATORCE Y TERRITORIOS ADYACENTES, SAN LUIS POTOSI, MÉXICO ONÉSIMO GONZÁLEZ COSTILLA1,2, JOAQUÍN GIMÉNEZ DE AZCÁRATE3, JOSÉ GARCÍA PÉREZ1 Y JUAN RogELIO AGUIRRE RIVERA1 1Universidad Autónoma de San Luis Potosí, Instituto de Investigación de Zonas Desérticas, Altair 200, Fraccionamiento El Llano, Apdo. postal 504, 78377 San Luis Potosí, México. 2Universidad Complutense de Madrid, Departamento de Biología Vegetal II, Facultad de Farmacia, Madrid, España. [email protected] 3Universidad de Santiago de Compostela, Departamento de Botánica, Escuela Politécnica Superior, 27002 Lugo, España. RESUMEN La Sierra de Catorce, localizada en el norte del estado de San Luis Potosí, reúne algunas de las principales cimas del Desierto Chihuahuense cuyas cotas superan los 3000 metros. Ello ha favorecido que la Sierra sea una importante área de diversificación de la flora y las fitocenosis de dicha ecorregión. A partir del estudio fitosociológico de la vegetación del territorio, que se está realizando desde 1999, se ha obtenido un catálogo preliminar de su flora. Hasta el momento la lista de plantas vasculares está conformada por 526 especies y cuatro taxa infraespecíficos, agrupados en 293 géneros y 88 familias. Las familias y géneros mejor representados son Asteraceae, Poaceae, Cactaceae, Fabaceae, Fagaceae y Lamiaceae, así como Quercus, Opuntia, Muhlenbergia, Salvia, Agave, Bouteloua y Dyssodia, respectivamente. Asimismo se señalan los tipos de vegetación representativos del área que albergan los diferentes taxa. Por último, con base en diferentes listas de flora amenazada, se identificaron las especies incluidas en alguna de las categorías reconocidas. Palabras clave: Desierto Chihuahuense, estudio fitosociológico, flora, flora ame- nazada, México, San Luis Potosí, Sierra de Catorce. -

Flaveria Bidentis (L.) Kuntze (Asteraceae), a Newly Naturalized Plant in Taiwan
林業研究季刊 30(4) : 23-詣 , 2008 23 蚯盔盤益 臺灣新歸化菊科植物-黃頂菊 曾彥學 1 劉靜愉 2 嚴新富 3 彭鏡毅 4 , 5 [摘要】本文首次報導原產北美洲南部,目前已歸化於台灣嘉義縣鱉鼓沿海附近的黃頂菊(菊科) , 描述其形態特徵、地理分布及生育地環境,並提供彩色圖片與線繪圖以資辨識 。 作者等於 1987 年 即已發現黃頂菊族群, 2008 年再進行調查時發現其野外族群穩定成長 。 黃頂菊為臺灣新歸化植物, 本屬亦為臺灣新記錄屬 。 [關鍵詞]菊科、黃頂菊、歸化植物、臺灣 Research paper Flaveria bidentis (L.) Kuntze (Asteraceae), a Newly Naturalized Plant in Taiwan Yen-Hsueh Tseng1 Chíng-Yu Líou2 Hsin-Fu Yen3 Ching-I Peng4,5 <Abstract) We document the naturalization of Flaveria bidentis (L.) Kuntze (Asteraceae) in southem Taiwan. This is one ofthe many cases ofNew World plants invading Taiwan. A taxonomic treatment, line drawings, and color photographs of this species 企om the wild are provided to aid in identification of this alien plant. The colony of F bidentis was first observed in Taiwan in1987. During our field survey in 2008 we witnessed that the wild populations has adapted to the coast of Chiayi County. (Key words] Asteraceae, Flaveria bident肉, Taxonomy, Naturalized plant, Taiwan 1.團立中興大學森林學系, 402 台中市南區團光路 250 號,台灣 Department ofForestry, National Chung-Hsing University, Taichung 402, Taiwan. 2 . 特有生物研究保育 中心棲地生態組, 552 南投縣集集鎮民生東路 1 號,台灣 Department ofHabitat and Ecology, Taiwan Endemic Species Research Institute , Nantou 552, Taiwan. 3. 國立自然科學博物館植物學組, 404 台中市館前路 1 號,臺 j彎 Department ofBotany, National Museum ofNatural Science, 1, Guancian Rd. , Taichung 404, Taiwan. 4 . 中央研究院生物多樣性研究中心, 115 台北市研究院路 2 段 128 號,台灣 Herbarium (HAST), Research Center for Biodiversity, Academia Sinica, Nankang, Taipei 115, Taiwan. 5 . 通訊作者: Email:[email protected] Corresponding author. EmaiJ : [email protected] 24 臺灣新歸化菊科植物-黃頂菊 INTRODUCTION phyllaries, with 2 additional small exterior ones. In recent years, many species of Asteraceae Receptacle small, glabrous. -
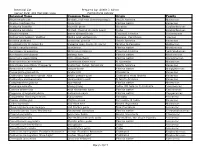
Botanical Name Common Name Origin Family
Botanical List Prepared by: Arielle J. Simon Corner Park: 401 Hampton Lane Horticultural Advisor Botanical Name Common Name Origin Family Abutilon pictum fireball, red vein flowering maple South America Malvaceae Acacia choriophylla cinnecord Florida native Fabaceae Acalypha hispida chenille plant Oceania Euphorbiaceae Acalypha pendula firetail, trailing chenille plant Cuba Euphorbiaceae Aloysia virgata sweet almond bush Tropical America Verbenaceae Anthurium hookeri 'Ruffles' bird's nest anthurium Guyana, Caribbean Araceae Arachis glabrata perennial peanut South America Fabaceae Arachnothryx leucophylla Panama rose, huele de noche Mexico to Panama Rubiaceae Ardisia escallonioides marlberry Florida native Myrsinaceae Asclepias curassavica Mexican milkweed Tropical America Asclepiadaceae Blechnum serrulatum swamp fern Florida native Blechnaceae Bourreria cassinifolia little strongback Florida native Boraginaceae Brachychiton acerifolius Australian flame tree N Australia Malvaceae Brunfelsia pauciflora 'Compacta' yesterday, today, tomorrow South America Fabaceae Byrsonima lucida locust-berry Florida native Malpighiaceae Caesalpinia granadillo bridal veil Venezuela Fabaceae Calliandra haematocephala 'Alba' white powder-puff Cultivated from Bolivia Fabaceae Calliandra surinamensis pink powder-puff N South America Fabaceae Calyptranthes pallens spicewood Florida native Myrtaceae Cananga odorata ylang-ylang India, SE Asia to N Australia Annonaceae Canella winterana wild cinnamon bark Florida native Canellaceae Capparis cynophallophora Jamaican -

Plant List: M.E. Depalma Park Picture Botanical Name Common Name Picture Botanical Name Common Name Salvia Coccinea Tropical
Plant List: M.E. DePalma Park Picture Botanical Name Picture Botanical Name Common Name Common Name Salvia coccinea Muhlenbergia capillaris Tropical Sage (red) pink Florida Muhly Grass Blooms: Year-round Blooms: Mid Summer - Fall Attracts Butterflies, Hummingbirds and Painted Buntings Florida Native Florida Native Psychotria nervosa Arachis glabrata Wild Coffee Perennial peanut Blooms: Spring-Summer Fruits: Fall Blooms: Year-round Berries are a food source for birds Native to Argentina, Brazil and Paraguay Male Butterflies need the alkaloids found in this plant, in order to mature sexually. Florida Native Callicarpa americana Gaillardia pulchella Beautyberry Blanket Flower Blooms: late Summer Fruits: Fall Blooms: Year-round Berries are a food source for birds Butterfly nectar plant Florida Native Florida Native Chrysobalanus icaco Stachytarpheta jamaicensis Cocoplum Blue Porterweed Blooms: Spring-Summer Fruits: Fall Blooms: Year-round Berries are a food source for birds Butterfly nectar plant Florida Native Florida Native Asclepias curassavica Pentas lanceolata Mexican Butterfly Weed, Blood-flower or Scarlet Milkweed Egyptian starflower Blooms: Year-round Blooms: Year-round Butterfly nectar plant for Butterflies especially Queens and These may be red, white, lavender, purple, or shades Monarchs of pink. Some are two-toned. All are extremely Monarch larval plant attractive to butterflies, and the red and dark pink varieties delight hummingbirds. Butterfly nectar plant Mimosa strigillosa Loropetalum Sensitive Plant Chinese Fringe Flower Blooms: Year-round Butterfly nectar plant Butterfly nectar plant Native to Japan and southeastern Asia including Florida Native groundcover southern China. Coreopsis_leavenworthii Coreopsis lanceolata Tickseed Lance leafed coreopsis Blooms: Year-round Butterfly nectar plant Butterfly nectar plant Florida Native Florida Native Lantana involucrata. -

Asteraceae: Astereae), an Endemic Shrub of the Galapagos Islands Nicole Genet Andrus Florida International University
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 7-24-2002 The origin, phylogenetics and natural history of darwiniothamnus (Asteraceae: Astereae), an endemic shrub of the Galapagos Islands Nicole Genet Andrus Florida International University DOI: 10.25148/etd.FI14032319 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Biology Commons Recommended Citation Andrus, Nicole Genet, "The origin, phylogenetics and natural history of darwiniothamnus (Asteraceae: Astereae), an endemic shrub of the Galapagos Islands" (2002). FIU Electronic Theses and Dissertations. 1290. https://digitalcommons.fiu.edu/etd/1290 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida THE ORIGIN, PHYLOGENETICS AND NATURAL HISTORY OF DARWINIOTHAMNUS (ASTERACEAE: ASTEREAE), AN ENDEMIC SHRUB OF THE GALAPAGOS ISLANDS A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in BIOLOGY by Nicole Genet Andrus 2002 To: Dean Arthur W. Herriott College of Arts and Sciences This thesis, written by Nicole Genet Andrus, and entitled The Origin, Phylogenetics and Natural History of Darwiniothamnus (Asteraceae: Astereae), an Endemic Shrub of the Galapagos Islands, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this thesis and recommend that it be approved. Alan Tye Susan Koptur Carl Lewis Javiefr acisco-Ortega, Major Professor Date of Defense: July 24, 2002 The thesis of Nicole Genet Andrus is approved. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3. -

Increasing Water Use Efficiency Along the C3 to C4 Evolutionary Pathway
Journal of Experimental Botany, Vol. 65, No. 13, pp. 3683–3693, 2014 doi:10.1093/jxb/eru205 Advance Access publication 23 May, 2014 This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details) RESEARCH PAPER Increasing water use efficiency along the 3C to C4 evolutionary pathway: a stomatal optimization perspective Danielle A. Way1,2,*, Gabriel G. Katul2,3, Stefano Manzoni4,5,6 and Giulia Vico4 1 Department of Biology, Western University, London, ON, Canada 2 Nicholas School of the Environment, Duke University, Durham, NC, USA 3 Department of Civil and Environmental Engineering, Duke University, Durham, NC, USA 4 Department of Crop Production Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden 5 Department of Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden 6 Department of Physical Geography and Quaternary Geology, Stockholm University, Stockholm, Sweden. Downloaded from * To whom correspondence should be addressed. E-mail: [email protected] Received 30 December 2013; Revised 8 April 2014; Accepted 15 April 2014 http://jxb.oxfordjournals.org/ Abstract C4 photosynthesis evolved independently numerous times, probably in response to declining atmospheric CO2 con- centrations, but also to high temperatures and aridity, which enhance water losses through transpiration. Here, the environmental factors controlling stomatal behaviour of leaf-level carbon and water exchange were examined across –1 –1 the evolutionary continuum from C3 to C4 photosynthesis at current (400 μmol mol ) and low (280 μmol mol ) atmos- pheric CO2 conditions. To this aim, a stomatal optimization model was further developed to describe the evolutionary at Duke University on August 8, 2014 continuum from C3 to C4 species within a unified framework. -

Performance of Native Florida Plants Under North Florida Conditions 1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. ENH1074 Performance of Native Florida Plants under North Florida Conditions 1 Jeffrey G. Norcini and James H. Aldrich 2 1. This document is ENH1074, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Original publication date November, 2007. Visit the EDIS Web Site at http://edis.ifas.ufl.edu. 2. Jeffrey G. Norcini, Associate Professor, native wildflower specialist and James H. Aldrich, Senior Biological Scientist, North Florida. Research & Education Center, Quincy, FL 32351. The Institute of Food and Agricultural Sciences (IFAS) is an Equal Opportunity Institution authorized to provide research, educational information and other services only to individuals and institutions that function with non-discrimination with respect to race, creed, color, religion, age, disability, sex, sexual orientation, marital status, national origin, political opinions or affiliations. U.S. Department of Agriculture, Cooperative Extension Service, University of Florida, IFAS, Florida A. & M. University Cooperative Extension Program, and Boards of County Commissioners Cooperating. Larry Arrington, Dean Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. Performance of Native Florida Plants under North Florida Conditions1 Jeffrey G. Norcini and James H. Aldrich2 Demand for native wildflowers and grasses has increased over the past 15-20 years as part of the surge in interest in use of native species, especially native plants that are adapted to local or regional conditions. Such native plants often are referred to as ecotypes. -

In Situ PHYTOREMEDIATION in MEXICO: a REVIEW
Artículo de Revisión Rev. Fitotec. Mex. Vol. 44 (2): 133 - 142, 2021 In situ PHYTOREMEDIATION IN MEXICO: A REVIEW FITORREMEDIACIÓN in situ EN MÉXICO: UNA REVISIÓN Cynthia Wong-Argüelles1, Candy Carranza-Álvarez2*, Angel J. Alonso-Castro3 y César A. Ilizaliturri-Hernández4 1Universidad Autónoma de San Luis Potosí (UASLP), Programas Multidisciplinarios de Posgrado en Ciencias Ambientales, San Luis Potosí, S.L.P., México. 2UASLP, Unidad Académica Multidisciplinaria Zona Huasteca, Cd. Valles, S.L.P., México. 3Universidad de Guanajuato, División de Ciencias Naturales y Exactas, Guanajuato, Guanajuato, México. 4 UASLP, Centro de Investigación Aplicada en Ambiente y Salud, San Luis Potosí, S.L.P., México. * Autor de correspondencia ([email protected]) SUMMARY contaminados por Pb. Las especies de plantas nativas deben estudiarse para establecer mecanismos de fitoextracción de metales y la interacción agua- In Mexico, contamination by potentially toxic elements in soil and water suelo-microorganismos para mejorar la eficiencia de la fitorremediación in represents important ecological and health problems. Plants capable of situ. La información aquí descrita tiene utilidad para planificar la remediación growing on anthropogenically-modified soils reflect their ability to adapt to de sitios contaminados por elementos potencialmente tóxicos en México y diverse environmental conditions. Most of the phytoremediation studies are para diferentes sitios del mundo. carried out under laboratory conditions, and only a few studies evaluate the phytoextraction capacity in situ. This review summarizes the information Palabras clave: Contaminación, fitorremediación, elementos obtained from scientific sources on in situ phytoremediation studies carried potencialmente tóxicos, plantas. out in Mexico. Eighty-five percent of the studies corresponded to sites contaminated with trace metals by mining activities. -

The Molecular Evolution of B-Carbonic Anhydrase in Flaveria
Journal of Experimental Botany, Vol. 62, No. 9, pp. 3071–3081, 2011 doi:10.1093/jxb/err071 Advance Access publication 15 March, 2011 REVIEW PAPER The molecular evolution of b-carbonic anhydrase in Flaveria Martha Ludwig* School of Biomedical, Biomolecular and Chemical Sciences, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia * To whom correspondence should be addressed. E-mail: [email protected] Received 17 November 2010; Accepted 21 February 2011 Downloaded from Abstract Limited information exists regarding molecular events that occurred during the evolution of C4 plants from their C3 ancestors. The enzyme b-carbonic anhydrase (CA; EC 4.2.1.1), which catalyses the reversible hydration of CO2,is present in multiple forms in C3 and C4 plants, and has given insights into the molecular evolution of the C4 pathway in the genus Flaveria. cDNAs encoding three distinct isoforms of b-CA, CA1–CA3, have been isolated and examined http://jxb.oxfordjournals.org/ from Flaveria C3 and C4 congeners. Sequence data, expression analyses of CA orthologues, and chloroplast import assays with radiolabelled CA precursor proteins from the C3 species F. pringlei Gandoger and the C4 species F. bidentis (L.) Kuntze have shown that both contain chloroplastic and cytosolic forms of the enzyme, and the potential roles of these isoforms are discussed. The data also identified CA3 as the cytosolic isoform important in C4 photosynthesis and indicate that the C4 CA3 gene evolved as a result of gene duplication and neofunctionalization, which involved mutations in coding and non-coding regions of the ancestral C3 CA3 gene. -

Response of an Invasive Plant, Flaveria Bidentis, to Nitrogen Addition: a Test of Form-Preference Uptake
Biol Invasions DOI 10.1007/s10530-016-1231-1 ORIGINAL PAPER Response of an invasive plant, Flaveria bidentis, to nitrogen addition: a test of form-preference uptake Chaohe Huangfu . Huiyan Li . Xinwei Chen . Hongmei Liu . Hui Wang . Dianlin Yang Received: 29 August 2015 / Accepted: 9 July 2016 Ó Springer International Publishing Switzerland 2016 15 ? Abstract Plants differ in their capacity to use higher N–NH4 recovery across biomass compo- various forms of nitrogen (N). Although previous nents than both co-occurring native plants, Amaran- studies have suggested invasive plants alter N avail- thus retroflexus and Eclipta prostrata. F. bidentis ability, few distinguish their responses to various demonstrated a strong preference for ammonium ? - forms and different concentrations of inorganic N. In (NH4 ) over nitrate (NO3 ) and captured at least 15 ? order to understand how plant preference for N affects twice the N–NH4 as the native plants. By compar- invasions, we tested the growth and physiological ison, the two native species showed no preferences for response of Flaveria bidentis, an invasive plant across the form of N. The greater above-ground biomass of F. north China, to different forms and concentrations of bidentis contributed to its higher 15N recovery. We inorganic N. Seedlings of F. bidentis were cultivated suggest that the ability of F. bidentis to respond in a mothproof screen house to determine if this rapidly to changes in the N pool, especially in invader benefits from increased or altered forms of N. ammonium, may confer a competitive advantage to 15 ? - N-labeled NH4 and NO3 were applied to the soil this species over native species.