Course of Early Neurologic Symptom Severity After Endovascular Treatment of Anterior Circulation Large Vessel Occlusion Stroke
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download the Manual in PDF Format
/ 5Pectrum HdaByte1M division of Sphere, Inc. 2061 Challenger Drive, Alameda, CA 94501 (415) 522-3584 solitaire royale concept and design by Brad Fregger. Macintosh version programmed by Brodie Lockard. Produced by Software Resources International. Program graphics for Macintosh version by Dennis Fregger. Manual for Macintosh version by Bryant Pong, Brad Fregger, Mark Johnson, Larry Throgmorton and Karen Sherman. Editing and Layout by Mark Johnson and Larry Throgmorton. Package design by Brad Fregger and Karen Sherman. Package artwork by Marty Petersen. If you have questions regarding the use of solitaire royale, or any of our other products, please call Spectrum HoloByte Customer Support between the hours of 9:00 AM and 5:00PM Pacific time, Monday through Friday, at the following number: (415) 522-1164 / or write to: rbJ Spectrum HoloByte 2061 Challenger Drive Alameda, CA 94501 Attn: Customer Support solitaire royale is a trademark of Software Resources International. Copyright © 1987 by Software Resources International. All rights reserved. Published by the Spectrum HoloByte division of Sphere, Inc. Spectrum HoloByte is a trademark of Sphere, Inc. Macintosh is a registered trademark of Apple Computer, Inc. PageMaker is a trademark of Aldus Corporation. Player's Guide FullPaint is a trademark of Ann Arbor Softworks, Inc. Helvetica and Times are registered trademarks of Allied Corporation. ITC Zapf Dingbats is a registered trademark of International Typeface Corporation. Contents Introduction .................................................................................. -

9/11 Report”), July 2, 2004, Pp
Final FM.1pp 7/17/04 5:25 PM Page i THE 9/11 COMMISSION REPORT Final FM.1pp 7/17/04 5:25 PM Page v CONTENTS List of Illustrations and Tables ix Member List xi Staff List xiii–xiv Preface xv 1. “WE HAVE SOME PLANES” 1 1.1 Inside the Four Flights 1 1.2 Improvising a Homeland Defense 14 1.3 National Crisis Management 35 2. THE FOUNDATION OF THE NEW TERRORISM 47 2.1 A Declaration of War 47 2.2 Bin Ladin’s Appeal in the Islamic World 48 2.3 The Rise of Bin Ladin and al Qaeda (1988–1992) 55 2.4 Building an Organization, Declaring War on the United States (1992–1996) 59 2.5 Al Qaeda’s Renewal in Afghanistan (1996–1998) 63 3. COUNTERTERRORISM EVOLVES 71 3.1 From the Old Terrorism to the New: The First World Trade Center Bombing 71 3.2 Adaptation—and Nonadaptation— ...in the Law Enforcement Community 73 3.3 . and in the Federal Aviation Administration 82 3.4 . and in the Intelligence Community 86 v Final FM.1pp 7/17/04 5:25 PM Page vi 3.5 . and in the State Department and the Defense Department 93 3.6 . and in the White House 98 3.7 . and in the Congress 102 4. RESPONSES TO AL QAEDA’S INITIAL ASSAULTS 108 4.1 Before the Bombings in Kenya and Tanzania 108 4.2 Crisis:August 1998 115 4.3 Diplomacy 121 4.4 Covert Action 126 4.5 Searching for Fresh Options 134 5. -
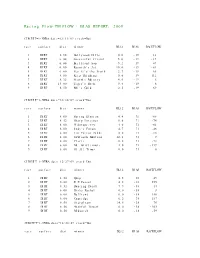
Racing Flow-TM FLOW + BIAS REPORT: 2009
Racing Flow-TM FLOW + BIAS REPORT: 2009 CIRCUIT=1-NYRA date=12/31/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 5.50 Hollywood Hills 0.0 -19 13 2 DIRT 6.00 Successful friend 5.0 -19 -19 3 DIRT 6.00 Brilliant Son 5.2 -19 47 4 DIRT 6.00 Raynick's Jet 10.6 -19 -61 5 DIRT 6.00 Yes It's the Truth 2.7 -19 65 6 DIRT 8.00 Keep Thinking 0.0 -19 -112 7 DIRT 8.32 Storm's Majesty 4.0 -19 6 8 DIRT 13.00 Tiger's Rock 9.4 -19 6 9 DIRT 8.50 Mel's Gold 2.5 -19 69 CIRCUIT=1-NYRA date=12/30/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.00 Spring Elusion 4.4 71 -68 2 DIRT 8.32 Sharp Instinct 0.0 71 -74 3 DIRT 6.00 O'Sotopretty 4.0 71 -61 4 DIRT 6.00 Indy's Forum 4.7 71 -46 5 DIRT 6.00 Ten Carrot Nikki 0.0 71 -18 6 DIRT 8.00 Sawtooth Moutain 12.1 71 9 7 DIRT 6.00 Cleric 0.6 71 -73 8 DIRT 6.00 Mt. Glittermore 4.0 71 -119 9 DIRT 6.00 Of All Times 0.0 71 0 CIRCUIT=1-NYRA date=12/27/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.50 Quip 4.5 -38 49 2 DIRT 6.00 E Z Passer 4.2 -38 255 3 DIRT 8.32 Dancing Daisy 7.9 -38 14 4 DIRT 6.00 Risky Rachel 0.0 -38 8 5 DIRT 6.00 Kaffiend 0.0 -38 150 6 DIRT 6.00 Capridge 6.2 -38 187 7 DIRT 8.50 Stargleam 14.5 -38 76 8 DIRT 8.50 Wishful Tomcat 0.0 -38 -203 9 DIRT 8.50 Midwatch 0.0 -38 -59 CIRCUIT=1-NYRA date=12/26/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 6.00 Papaleo 7.0 108 129 2 DIRT 6.00 Overcommunication 1.0 108 -72 3 DIRT 6.00 Digger 0.0 108 -211 4 DIRT 6.00 Bryan Kicks 0.0 108 136 5 DIRT 6.00 We Get It 16.8 108 129 6 DIRT 6.00 Yawanna Trust 4.5 108 -21 7 DIRT 6.00 Smarty Karakorum 6.5 108 83 8 DIRT 8.32 Almighty Silver 18.7 108 133 9 DIRT 8.32 Offlee Cool 0.0 108 -60 CIRCUIT=1-NYRA date=12/13/09 track=Dot race surface dist winner BL12 BIAS RACEFLOW 1 DIRT 8.32 Crafty Bear 3.0 -158 -139 2 DIRT 6.00 Cheers Darling 0.5 -158 61 3 DIRT 6.00 Iberian Gate 3.0 -158 154 4 DIRT 6.00 Pewter 0.5 -158 8 5 DIRT 6.00 Wolfson 6.2 -158 86 6 DIRT 6.00 Mr. -

Violin Syllabus / 2013 Edition
VVioliniolin SYLLABUS / 2013 EDITION SYLLABUS EDITION © Copyright 2013 The Frederick Harris Music Co., Limited All Rights Reserved Message from the President The Royal Conservatory of Music was founded in 1886 with the idea that a single institution could bind the people of a nation together with the common thread of shared musical experience. More than a century later, we continue to build and expand on this vision. Today, The Royal Conservatory is recognized in communities across North America for outstanding service to students, teachers, and parents, as well as strict adherence to high academic standards through a variety of activities—teaching, examining, publishing, research, and community outreach. Our students and teachers benefit from a curriculum based on more than 125 years of commitment to the highest pedagogical objectives. The strength of the curriculum is reinforced by the distinguished College of Examiners—a group of fine musicians and teachers who have been carefully selected from across Canada, the United States, and abroad for their demonstrated skill and professionalism. A rigorous examiner apprenticeship program, combined with regular evaluation procedures, ensures consistency and an examination experience of the highest quality for candidates. As you pursue your studies or teach others, you become not only an important partner with The Royal Conservatory in the development of creativity, discipline, and goal- setting, but also an active participant, experiencing the transcendent qualities of music itself. In a society where our day-to-day lives can become rote and routine, the human need to find self-fulfillment and to engage in creative activity has never been more necessary. -

Overview of State Visits by Queen Beatrix
Overview of state visits by Queen Beatrix 24-25 January 2013: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to President Tony Tan Keng Yam, Singapore 21-22 January 2013: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to Sultan and Yang Di-Pertuan (head of state) Haji Hassanal Bolkiah, Brunei Darussalam 20-22 March 2012: Queen Beatrix to Grand Duke Henri and Grand Duchess Maria Teresa, Luxembourg 10-12 January 2012: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to Sultan Qaboos bin Said al-Said, Oman 8-9 January 2012: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to President Khalifa bin Zayed bin Sultan Al Nahayan, United Arab Emirates 12-15 April 2011: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to President and Mrs Wulff, Germany 9-10 March 2011: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to Emir Hamad bin Khalifa Al-Thani, Qatar 6-8 March 2011: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to Sultan Qaboos bin Said al-Said, Oman. Because of unrest in the Middle East, this was changed to a private visit. 1-3 June 2010: Queen Beatrix to King Harald V and Queen Sonja, Norway 3-6 November 2009: Queen Beatrix, accompanied by the Prince of Orange and Princess Máxima, to President Felipe Calderón, Mexico 24-26 June 2008: Queen Beatrix to President Adamkus and Mrs Adamkiené, Lithuania 14-16 May 2008: Queen Beatrix to President and Mrs Ilves, Estonia 24-27 October -
Saddlebred Sidewalk List.Docx
CH 20TH CENTURY FOX A CHAMPAGNE TOAST A CONVERSATION PIECE A DAY IN THE SUN A DAY IN THE SUN A KINDLING FLAME A LIKELY STORY A LITTLE IMAGINATION A LOTTA LOVIN' CH A MAGIC SPELL A MAGICAL CHOICE A NOTCH ABOVE A PLACE IN THE HEART A RARITY A RICH GIRL A ROYAL QUEST A ROYAL QUEST A SENSATION A SENSATIONAL FOX A SONG IN MY HEART A SPECIAL EVENT A SPECIAL SURPRISE CH A SUPREME SURPRISE CH A SWEET TREAT CH A TOUCH OF CHAMPAGNE CH A TOUCH OF CHAMPAGNE A TOUCH OF PIZZAZZ A TOUCH OF RADIANCE A TOUCH OF TENDERNESS A TRADITION CH A TRAVELIN' MAN A WINNING WONDER ABIE'S IRISH ROSE ABOVE BOARD ABRACADABRA ABSOLUTELY A LADY ABSOLUTELY COURAGEOUS CH ABSOLUTELY EXQUISITE CH ABSOLUTELY FABULOUS ABSOLUTELY NO LIMITS ACCELERATOR ACE HIGH DUKE ACES AND EIGHTS ACE'S FLASHING GENIUS ACE HIGH DUKE ACES AND EIGHTS ACE'S FLASHING GENIUS ACE'S QUEEN ROSE ACE'S REFRESHMENT TIME ACE'S SOARING SPIRIIT ACE'S SWEET LAVENDER ACE'S TEMPTATION ACTIVE SERVICE ADF STRICTLY CLASS ADMIRAL FOX ADMIRAL'S AVENGER ADMIRAL'S BLACK FEATHER ADMIRAL'S COMMAND CH ADMIRAL'S FLEET CH ADMIRAL'S MARK CH ADMIRAL'S MARK ADMIRAL'S SHIPMATE CH ADVANTAGE ME CH ADVANTAGE ME AFFAIR OF THE HEART "JAKE" AFLAME AGLOW AGLOW AHEAD OF THE CLASS AIN'T MISBEHAVIN AIN'T MISBEHAVIN AIR OF ELEGANCE CH ALETHA STONEWALL ALEXANDER THE GREAT ALEXANDER'S EMERAUDE ALEXANDER'S EMERAUDE ALFIE ALICE LOU'S DENMARK CH ALISON MACKENZIE CH ALISON MACKENZIE CH ALL AMERICAN BOY ALL DRESSED UP ALL NIGHT LONG ALL ROSES ALL SHOW ALL THE MONEY ALLEGORY ALLIE COME LATELY "BAH" ALL'S CLEAR ALLSWEET ALLURING LADY ALONG CAME A SPIDER ALTERED IMAGE ALTON BELLE ALONG CAME A SPIDER ALTERED IMAGE ALTON BELLE CH ALVIN'S MR. -

The Making of Sultan Süleyman: a Study of Process/Es of Image-Making and Reputation Management
THE MAKING OF SULTAN SÜLEYMAN: A STUDY OF PROCESS/ES OF IMAGE-MAKING AND REPUTATION MANAGEMENT by NEV ĐN ZEYNEP YELÇE Submitted to the Institute of Social Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy in History Sabancı University June, 2009 © Nevin Zeynep Yelçe 2009 All Rights Reserved To My Dear Parents Ay şegül and Özer Yelçe ABSTRACT THE MAKING OF SULTAN SÜLEYMAN: A STUDY OF PROCESS/ES OF IMAGE-MAKING AND REPUTATION MANAGEMENT Yelçe, Nevin Zeynep Ph.D., History Supervisor: Metin Kunt June 2009, xv+558 pages This dissertation is a study of the processes involved in the making of Sultan Süleyman’s image and reputation within the two decades preceding and following his accession, delineating the various phases and aspects involved in the making of the multi-layered image of the Sultan. Handling these processes within the framework of Sultan Süleyman’s deeds and choices, the main argument of this study is that the reputation of Sultan Süleyman in the 1520s was the result of the convergence of his actions and his projected image. In the course of this study, main events of the first ten years of Sultan Süleyman’s reign are conceptualized in order to understand the elements employed first in making a Sultan out of a Prince, then in maintaining and enhancing the sultanic image and authority. As such, this dissertation examines the rhetorical, ceremonial, and symbolic devices which came together to build up a public image for the Sultan. Contextualized within a larger framework in terms of both time and space, not only the meaning and role of each device but the way they are combined to create an image becomes clearer. -

Solitaire: Man Versus Machine
Solitaire: Man Versus Machine Xiang Yan∗ Persi Diaconis∗ Paat Rusmevichientong† Benjamin Van Roy∗ ∗Stanford University {xyan,persi.diaconis,bvr}@stanford.edu †Cornell University [email protected] Abstract In this paper, we use the rollout method for policy improvement to an- alyze a version of Klondike solitaire. This version, sometimes called thoughtful solitaire, has all cards revealed to the player, but then follows the usual Klondike rules. A strategy that we establish, using iterated roll- outs, wins about twice as many games on average as an expert human player does. 1 Introduction Though proposed more than fifty years ago [1, 7], the effectiveness of the policy improve- ment algorithm remains a mystery. For discounted or average reward Markov decision problems with n states and two possible actions per state, the tightest known worst-case upper bound in terms of n on the number of iterations taken to find an optimal policy is O(2n/n) [9]. This is also the tightest known upper bound for deterministic Markov de- cision problems. It is surprising, however, that there are no known examples of Markov decision problems with two possible actions per state for which more than n + 2 iterations are required. A more intriguing fact is that even for problems with a large number of states – say, in the millions – an optimal policy is often delivered after only half a dozen or so iterations. In problems where n is enormous – say, a googol – this may appear to be a moot point because each iteration requires Ω(n) compute time. In particular, a policy is represented by a table with one action per state and each iteration improves the policy by updating each entry of this table. -
![The Compleat Works of Nostradamus -=][ Compiled and Entered in PDF Format by Arcanaeum: 2003 ][=](https://docslib.b-cdn.net/cover/3323/the-compleat-works-of-nostradamus-compiled-and-entered-in-pdf-format-by-arcanaeum-2003-2343323.webp)
The Compleat Works of Nostradamus -=][ Compiled and Entered in PDF Format by Arcanaeum: 2003 ][=
The Compleat Works of Nostradamus -=][ compiled and entered in PDF format by Arcanaeum: 2003 ][=- Table of Contents: Preface Century I Century II Century III Century IV Century V Century VI Century VII Century VIII Century IX Century X Epistle To King Henry II Pour les ans Courans en ce Siecle (roughly translated: for the years’ events in this century) Almanacs: 1555−1563 Note: Many of these are written in French with the English Translation directly beneath them. Preface by: M. Nostradamus to his Prophecies Greetings and happiness to César Nostradamus my son Your late arrival, César Nostredame, my son, has made me spend much time in constant nightly reflection so that I could communicate with you by letter and leave you this reminder, after my death, for the benefit of all men, of which the divine spirit has vouchsafed me to know by means of astronomy. And since it was the Almighty's will that you were not born here in this region [Provence] and I do not want to talk of years to come but of the months during which you will struggle to grasp and understand the work I shall be compelled to leave you after my death: assuming that it will not be possible for me to leave you such [clearer] writing as may be destroyed through the injustice of the age [1555]. The key to the hidden prediction which you will inherit will be locked inside my heart. Also bear in mind that the events here described have not yet come to pass, and that all is ruled and governed by the power of Almighty God, inspiring us not by bacchic frenzy nor by enchantments but by astronomical assurances: predictions have been made through the inspiration of divine will alone and the spirit of prophecy in particular. -

New Age, Vol. 17, No. 4, May 27, 1915
NOTES OF THE WEEK . MORE LETTERS TO MY NEPHEW. By Anthony Farley CURRENTCANT . LESYA UKRAINKA’S “BABYLONIAN CAPTIVITY.” FOREIGNAFFAIRS. By S. Verdad . (Trans. by S. Wolska and C. E. Bechhofer) MILITARY NOTES. By Romney . PROBLEMS OF CONFLICT. By Everard G. Gilbert- TOWARDSNATIONAL GUILDS. By “National Guildsmen" Cooper . VIEWS AND REVIEWS: BACK TO THE VEDAS. By A. E. R. ASPECTS OF THE GUILDIDEA-IV. By Ivor Brown PASTICHE. By J. A. M. A., 1’. V. C., P. Selver, South AFRICAN ECHOES . Ruth Pitter, B. M., Vectis . WAR ANDSOLIDARITY. By Ramiro de Maeztu . LETTERS TO THE EDITOR from A. E. R., Evan IMPRESSIONSOF PARIS. By Alice Morning . Morgan, S. Reeve, A. Stratton, Fred H. READERS AND WRITERS. By R. H. C. Gorle, F. W. O’Connor . NOTES OF THE WEEK. revolutionaryreconstruction of it? It certainly seems to us insufficient, and, what is more, we do not believe that THE worst of having no House of Commons is that a soul in the country will believe it. Even supposing except for the Front Benches and their favoured Press that this quartette of men should be so unpatriotic as to and City men, nobody in the country knows what is continue their personal quarrels at the cost of the lives going on. We are like the crowd on the outermost of thousands of their countrymen-to say nothing of edges of a fight on the issue of which, nevertheless, our the country itself-it is inconceivable that a Cabinet, very lives depend. Now and then a rift in the throng having no worse troubles on its hands, could find no pressing about the combatants reveals us something. -

The Dictionary Legend
THE DICTIONARY The following list is a compilation of words and phrases that have been taken from a variety of sources that are utilized in the research and following of Street Gangs and Security Threat Groups. The information that is contained here is the most accurate and current that is presently available. If you are a recipient of this book, you are asked to review it and comment on its usefulness. If you have something that you feel should be included, please submit it so it may be added to future updates. Please note: the information here is to be used as an aid in the interpretation of Street Gangs and Security Threat Groups communication. Words and meanings change constantly. Compiled by the Woodman State Jail, Security Threat Group Office, and from information obtained from, but not limited to, the following: a) Texas Attorney General conference, October 1999 and 2003 b) Texas Department of Criminal Justice - Security Threat Group Officers c) California Department of Corrections d) Sacramento Intelligence Unit LEGEND: BOLD TYPE: Term or Phrase being used (Parenthesis): Used to show the possible origin of the term Meaning: Possible interpretation of the term PLEASE USE EXTREME CARE AND CAUTION IN THE DISPLAY AND USE OF THIS BOOK. DO NOT LEAVE IT WHERE IT CAN BE LOCATED, ACCESSED OR UTILIZED BY ANY UNAUTHORIZED PERSON. Revised: 25 August 2004 1 TABLE OF CONTENTS A: Pages 3-9 O: Pages 100-104 B: Pages 10-22 P: Pages 104-114 C: Pages 22-40 Q: Pages 114-115 D: Pages 40-46 R: Pages 115-122 E: Pages 46-51 S: Pages 122-136 F: Pages 51-58 T: Pages 136-146 G: Pages 58-64 U: Pages 146-148 H: Pages 64-70 V: Pages 148-150 I: Pages 70-73 W: Pages 150-155 J: Pages 73-76 X: Page 155 K: Pages 76-80 Y: Pages 155-156 L: Pages 80-87 Z: Page 157 M: Pages 87-96 #s: Pages 157-168 N: Pages 96-100 COMMENTS: When this “Dictionary” was first started, it was done primarily as an aid for the Security Threat Group Officers in the Texas Department of Criminal Justice (TDCJ). -

Cruel Necessity: the English Civil Wars 1640 to 1653 Designer’S Notes
Cruel Necessity: The English Civil Wars 1640 to 1653 Designer’s Notes Steve Carey and Tim Porter seemingly players to engage in exciting tactical spent two years’ worth of GMT West battles and, although usually indecisive, Cruel Necessity and Pacificon conventions to extended garner benefits from any great success Designer’s Notes by John Welch playtesting of this early version of the achieved in doing so. The systems for the Cruel Necessity is my third States of game. They offered countless design mini-game had to be tight and relatively SiegeTM series game. I actually nailed ideas and, most importantly, kept me quick-and-clean to not slow gameplay on down the design in November of 2009 motivated to continue improving Cruel the main map. after designing Levee en Masse and Keep Necessity. Barely-trained forces fought the initial Up The Fire! My feeling is that each In January 2012, Cruel Necessity was battles of the English Civil Wars, with a game must be different from its selected to be the “new game” in a GMT slight advantage going to the King’s men. predecessor, so with Levee I added P500 States of SiegeTM three-pack dubbed The turning point occurred when Political tracks, Liberation, and near- Revolt and Revolution. It was exciting to Cromwell demanded that “Godly” troops perpetual Unrest in Paris; in Fire! it was watch the numbers climb and pass the be raised and, more importantly, trained. the ‘double game’ of tactical combat at 500 preorder threshold. At this point, I This New Model Army, along with a the compound and then to strategically felt the game was in pretty good shape – disciplined cavalry, were the keys to organize and lead the Relief Army to three years of work really showed.