Field Release of the Arundo Wasp, Tetramesa Romana
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Density, Distribution and Habitat Requirements for the Ozark Pocket Gopher (Geomys Bursarius Ozarkensis)
DENSITY, DISTRIBUTION AND HABITAT REQUIREMENTS FOR THE OZARK POCKET GOPHER (Geomys bursarius ozarkensis) Audrey Allbach Kershen, B. S. Thesis Prepared for the Degree of MASTER OF SCIENCE UNIVERSITY OF NORTH TEXAS May 2004 APPROVED: Kenneth L. Dickson, Co-Major Professor Douglas A. Elrod, Co-Major Professor Thomas L. Beitinger, Committee Member Sandra L. Terrell, Interim Dean of the Robert B. Toulouse School of Graduate Studies Kershen, Audrey Allbach, Density, distribution and habitat requirements for the Ozark pocket gopher (Geomys bursarius ozarkensis). Master of Science (Environmental Science), May 2004, 67 pp., 6 tables, 6 figures, 69 references. A new subspecies of the plains pocket gopher (Geomys bursarius ozarkensis), located in the Ozark Mountains of north central Arkansas, was recently described by Elrod et al. (2000). Current range for G. b. ozarkensis was established, habitat preference was assessed by analyzing soil samples, vegetation and distance to stream and potential pocket gopher habitat within the current range was identified. A census technique was used to estimate a total density of 3, 564 pocket gophers. Through automobile and aerial survey 51 known fields of inhabitance were located extending the range slightly. Soil analyses indicated loamy sand as the most common texture with a slightly acidic pH and a broad range of values for other measured soil parameters and 21 families of vegetation were identified. All inhabited fields were located within an average of 107.2m from waterways and over 1,600 hectares of possible suitable habitat was identified. ACKNOWLEDGMENTS Appreciation is extended to the members of my committee, Dr. Kenneth Dickson, Dr. Douglas Elrod and Dr. -

Evaluation of the Combustion Characteristics of Four Perennial Energy Crops (Arundo Donax, Cynara Cardunculus, Miscanthus X Giganteus and Panicum Virgatum)
2nd World Conference on Biomass for Energy, Industry and Climate Protection, 10-14 May 2004, Rome, Italy EVALUATION OF THE COMBUSTION CHARACTERISTICS OF FOUR PERENNIAL ENERGY CROPS (ARUNDO DONAX, CYNARA CARDUNCULUS, MISCANTHUS X GIGANTEUS AND PANICUM VIRGATUM) Jonas Dahl & Ingwald Obernberger Institute of Resource Efficient and Sustainable Systems, Graz University of Technology, Inffeldgasse 25, A - 8010 Graz, Austria, Tel.: +43 (0)316 481300, Fax: +43 (0)316 481300 4; E-mail: [email protected] ABSTRACT: The perennial crops giant reed, switchgrass, miscanthus and cardoon were investigated in laboratory- scale and pilot-scale combustion test runs. Laboratory-scale test runs were conducted in a fixed bed pot reactor monitoring the temperature in the fuel bed and the release of gaseous components while pilot-scale test runs where conducted in a 150 kWth rotating grate fired combustion plant measuring formed emissions of CO, NOX, SO2, HCl, and particulates as well as performing deposit probe measurements. The results revealed that the high concentration of ash and slag forming elements such as Si, K and Ca cause severe problems regarding slagging if not specially considered during combustion. Moreover, high concentrations of N are another challenge regarding measures in order to avoid high emissions of NOx. Moreover, also HCl and SO2 emissions are considerably higher compared to wood fuels due to the higher concentrations of Cl and S in these fuels. Keywords: biomass characteristics, biomass conversion, cynara cardonculus, giant reed, switchgrass, miscanthus 1 INTRODUCTION combustion unit (nominal boiler capacity 150 kWth) were carried out with larger amounts (2-4 tons) of Due to the limited availability of wood fuels, in pelletised switch grass and chopped giant reed and southern Europe, high yield energy crops could give an chopped miscanthus (MI). -

Advanced Biofuel Policies in Select Eu Member States: 2018 Update
© INTERNATIONAL COUNCIL ON CLEAN TRANSPORTATION POLICY UPDATE NOVEMBER 2018 ADVANCED BIOFUEL POLICIES IN SELECT EU MEMBER STATES: 2018 UPDATE This policy update provides details on the latest measures that select European ICCT POLICY UPDATES Union (EU) member states, namely Denmark, Germany, Italy, the Netherlands, SUMMARIZE Sweden, and the United Kingdom, are taking to support advanced alternative fuels. REGULATORY AND OTHER EU POLICY BACKGROUND DEVELOPMENTS In 2018, the European Union (EU) set its climate and energy objectives for 2030. RELATED TO CLEAN They included a greenhouse gas (GHG) reduction of at least 40% and a minimum of a TRANSPORTATION 32% share of renewable energy consumption across all sectors.1 GHG emissions in the WORLDWIDE. European transportation sector have declined by only 3.8% since 2008, compared to an 18% decrease, or more, in all other sectors, indicating that the decarbonization of transportation should be a priority for the future.2 Biofuels are one of the options considered to increase renewable energy and decrease the carbon intensity of the transportation sector. Through the use of directives and national legislation, the EU has incentivized both the adoption of conventional food-based biofuels and advanced biofuels, which are made from non-food feedstocks. Such incentives date to 2009, when the EU Renewable Energy Directive (RED) mandated that by 2020, 10% of energy used in the transportation sector should come from renewable energy sources (RES).3 In 2015, the RED was 1 Jacopo Giuntoli, Final recast Renewable Energy Directive for 2021-2030 in the European Union, (ICCT: Washington, DC, 2018), https://www.theicct.org/publications/final-recast-renewable-energy-directive- 2021-2030-european-union 2 EUROSTAT (Greenhouse gas emissions by source sector (env_air_gge), accessed November 2018), https://ec.europa.eu/eurostat. -

Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America North of Mexico with a Key to Species
Zootaxa 2835: 30–40 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America north of Mexico with a key to species J. E. McPHERSON1, RICHARD J. PACKAUSKAS2, ROBERT W. SITES3, STEVEN J. TAYLOR4, C. SCOTT BUNDY5, JEFFREY D. BRADSHAW6 & PAULA LEVIN MITCHELL7 1Department of Zoology, Southern Illinois University, Carbondale, Illinois 62901, USA. E-mail: [email protected] 2Department of Biological Sciences, Fort Hays State University, Hays, Kansas 67601, USA. E-mail: [email protected] 3Enns Entomology Museum, Division of Plant Sciences, University of Missouri, Columbia, Missouri 65211, USA. E-mail: [email protected] 4Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Illinois 61820, USA. E-mail: [email protected] 5Department of Entomology, Plant Pathology, & Weed Science, New Mexico State University, Las Cruces, New Mexico 88003, USA. E-mail: [email protected] 6Department of Entomology, University of Nebraska-Lincoln, Panhandle Research & Extension Center, Scottsbluff, Nebraska 69361, USA. E-mail: [email protected] 7Department of Biology, Winthrop University, Rock Hill, South Carolina 29733, USA. E-mail: [email protected] Abstract A review of Acanthocephala of America north of Mexico is presented with an updated key to species. A. confraterna is considered a junior synonym of A. terminalis, thus reducing the number of known species in this region from five to four. New state and country records are presented. Key words: Coreidae, Coreinae, Acanthocephalini, Acanthocephala, North America, review, synonymy, key, distribution Introduction The genus Acanthocephala Laporte currently is represented in America north of Mexico by five species: Acan- thocephala (Acanthocephala) declivis (Say), A. -

High-Throughput Sequencing of Six Bamboo Chloroplast Genomes: Phylogenetic Implications for Temperate Woody Bamboos (Poaceae: Bambusoideae)
High-Throughput Sequencing of Six Bamboo Chloroplast Genomes: Phylogenetic Implications for Temperate Woody Bamboos (Poaceae: Bambusoideae) Yun-Jie Zhang1,2,3., Peng-Fei Ma1,2,3., De-Zhu Li1,2* 1 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, People’s Republic of China, 2 Plant Germplasm and Genomics Center, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, People’s Republic of China, 3 Graduate University of Chinese Academy of Sciences, Beijing, People’s Republic of China Abstract Background: Bambusoideae is the only subfamily that contains woody members in the grass family, Poaceae. In phylogenetic analyses, Bambusoideae, Pooideae and Ehrhartoideae formed the BEP clade, yet the internal relationships of this clade are controversial. The distinctive life history (infrequent flowering and predominance of asexual reproduction) of woody bamboos makes them an interesting but taxonomically difficult group. Phylogenetic analyses based on large DNA fragments could only provide a moderate resolution of woody bamboo relationships, although a robust phylogenetic tree is needed to elucidate their evolutionary history. Phylogenomics is an alternative choice for resolving difficult phylogenies. Methodology/Principal Findings: Here we present the complete nucleotide sequences of six woody bamboo chloroplast (cp) genomes using Illumina sequencing. These genomes are similar to those of other grasses and rather conservative in evolution. We constructed a phylogeny of Poaceae from 24 complete cp genomes including 21 grass species. Within the BEP clade, we found strong support for a sister relationship between Bambusoideae and Pooideae. In a substantial improvement over prior studies, all six nodes within Bambusoideae were supported with $0.95 posterior probability from Bayesian inference and 5/6 nodes resolved with 100% bootstrap support in maximum parsimony and maximum likelihood analyses. -

Phylogenetic Analysis of Eurytominae (Chalcidoidea: Eurytomidae) Based on Morphological Characters
Blackwell Publishing LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082© 2007 The Linnean Society of London? 2007 1513 441510 Original Article PHYLOGENETIC ANALYSIS OF EURYTOMINAEH. LOTFALIZADEH ET AL. Zoological Journal of the Linnean Society, 2007, 151, 441–510. With 212 figures Phylogenetic analysis of Eurytominae (Chalcidoidea: Eurytomidae) based on morphological characters HOSSEINALI LOTFALIZADEH1, GÉRARD DELVARE2* and JEAN-YVES RASPLUS2 1Plant Pests and Diseases Research Institute, Evin, Tehran 19395–1454, Iran 2CIRAD – INRA, Centre de Biologie et de Gestion des Populations (CBGP), Campus International de Baillarguet, CS 30 016, F-34988 Montferrier-sur-Lez, France Received February 2006; accepted for publication December 2006 A phylogenetic study of the Eurytominae (Hymenoptera: Chalcidoidea) treating 178 taxa and based on 150 mor- phological characters is given. Several cladograms using the complete species sample, but obtained with different weightings, are presented. Local studies were also carried out to provide possible alternate topologies. The deep nodes of the trees were unstable and were never supported, but most of the superficial nodes were stable and robust. The results therefore provide support for a generic classification of the subfamily. The large genus Eurytoma – which includes about half of the described species of the subfamily – proved to be polyphyletic, and is redefined in a nar- rowed sense using putative synapomorphies. Bruchophagus and Prodecatoma were similarly redefined. The genera Philolema and Aximopsis are reconsidered and defined in a broader concept. A number of the species presently included in Eurytoma were transferred to these genera. Finally, 22 new generic synonymies are proposed and 33 spe- cies are transferred. The study also demonstrates that the Eurytomidae are polyphyletic. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Hazardous Fuels Management in Subtropical Pine Flatwoods and Tropical Pine Rocklands Joseph J
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln JFSP Research Project Reports U.S. Joint Fire Science Program 2007 Fire Managers Field Guide: Hazardous Fuels Management in Subtropical Pine Flatwoods and Tropical Pine Rocklands Joseph J. O'Brien USDA Forest Service Kathyryn A. Mordecai USDA Forest Service Leslie Wolcott USDA Forest Service James Snyder USGS Kenneth Outcalt USDA Forest Service Follow this and additional works at: http://digitalcommons.unl.edu/jfspresearch Part of the Forest Biology Commons, Forest Management Commons, Natural Resources and Conservation Commons, Natural Resources Management and Policy Commons, Other Environmental Sciences Commons, Other Forestry and Forest Sciences Commons, Sustainability Commons, and the Wood Science and Pulp, Paper Technology Commons O'Brien, Joseph J.; Mordecai, Kathyryn A.; Wolcott, Leslie; Snyder, James; and Outcalt, Kenneth, "Fire Managers Field Guide: Hazardous Fuels Management in Subtropical Pine Flatwoods and Tropical Pine Rocklands" (2007). JFSP Research Project Reports. 101. http://digitalcommons.unl.edu/jfspresearch/101 This Article is brought to you for free and open access by the U.S. Joint Fire Science Program at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in JFSP Research Project Reports by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Fire Managers Field Guide: Hazardous Fuels Management in Subtropical Pine Flatwoods and Tropical Pine Rocklands FFFiiinnnaaalll RRReeepppooorrrttt -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Designing W Grasses Complete Notes
DESIGNING W/ GRASSES: SLIDESHOW NAMES TONY SPENCER Google search botanical plant names or visit Missouri Botanical Garden site for more info: 1. Pennisetum alopecuroides + Sanguisorba + Molinia arundinacea ‘Transparent’ 2. Pennisetum alopecuroides + Aster + Molinia arundinacea ‘Transparent’ 3. Calamagrostis x. acutiflora ‘Karl Foerster’ + Panicum ‘Shenandoah’ 4. Helianthus pauciflorus – Photo Credit: Chris Helzer 5. Nassella tenuissima + Echinacea simulata + Monarda bradburiana 6. Hordeum jubatum + Astilbe 7. Deschampsia cespitosa + Helenium autumnale 8. Calamagrostis brachytricha + Miscanthus sinensis + Cimicifuga atropurpurea 9. Sporobolus heterlolepis + Echinacea pallida 10. Panicum virgatum + Echinacea pallida + Monarda + Veronica 11. Molinia arundinacea ‘Transparent + Sanguisorba officinalis 12. Bouteloua gracilis 13. Calamagrostis brachytricha + Helenium autumnale 14. Peucedanum verticillare 15. Anemone ‘Honorine Jobert’ 2016 Perennial Plant of the Year 16. Miscanthus sinsensis 17. Calamagrostis brachytricha 18. Molinia caerulea + Calamagrostis ‘Karl Foerster’ 19. Calamagrostis ‘Karl Foerster’ + Lythrum alatum + Parthenium integrafolium 20. Panicum virgatum ‘Shenandoah’ 21. Bouteloua gracilis + Echinacea ‘Kim’s Knee High’ + Salvia nemorosa 22. Baptisia alba 23. Calamagrostis ‘Karl Foerster’ in Hummelo meadow planting 24. Panicum amarum ‘Dewey Blue’ + Helenium autumnale 25. Deschampsia cespitosa 26. Echinacea purpurea seedheads 27. Calamagrostis brachytricha + Calamagrostis ‘Karl Foerster’ + Echinacea + Veronicastrum + Eupatorium -

Hymenoptera: Chalcidoidea
Madras Agric. J., 2019; doi:10.29321/MAJ 2019.000254 RESEARCH ARTICLE Comparision of Eurytomidae and Eupelmidae (Hymenoptera: Chalcidoidea) diversity from three rice growing zones of Tamil Nadu Alfred Daniel, J*1, Ramaraju, K2, Poorani, J3 and Nikhil, K4 1*,2Department of Agricultural Entomology, Tamil Nadu Agricultural University, Coimbatore - 641 003 3National Research Centre for Banana, Trichy - 620 102 4Western Ghat Field Research Centre, Zoological Survey of India, Calicut - 673 006 ABSTRACT Rice inhabiting Eurytomidae and Eupelmidae (Hymenoptera; Chalcidoidea) were collected from the western zone, Cauvery delta zone and high rainfall Received : 13th May, 2019 zone of Tamil Nadu during 2015-16. Collected Eurytomidae (105 individuals) Revised : 30th May, 2019 and Eupelmidae (81 individuals) comprised 8 genera and 12 species. Accepted : 30th May, 2019 Neobepharata sp. (Eurytomidae) and Mesocomys sp. (Eupelmidae) were the most abundant fauna among all the species observed with the relative abundance of 24.0 and 50.6 per cent respectively. Keywords: Diversity, Hymenopterans, Parasitoids, Eurytomidae, Eupelmidae, Rice Ecosystem. INTRODUCTION associated with rice ecosystem is poorly studied and far from satisfaction especially in Tamil Nadu. Rice fields have unique characteristics that make Additional knowledge on diversity, taxonomy them ideal grounds for diverse biological organisms and biology is of potential practical value in rice (Heckman, 1979; Fritz et al., 2011). Insect pests insect pest management. Globally only 7 species are a the major threat in rice production. More each of Eupelmidae and Eurytomidae have been than 800 species of insects are known to infest recorded in rice (Dey et al., 1999). From Tamil rice, of which about 20 species are of economic Nadu Anastatus coimbatorensis Girault alone has importance (Pathak and Dhaliwal, 1981). -
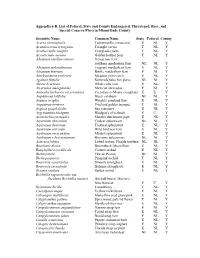
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var.