New Zealand Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Once-Daily Skeletal Muscle Relaxant in SA
NEWS Once-daily skeletal muscle relaxant in SA Myprocam (cyclobenzaprine with spine-related disorders and other 8. What are the classes of muscle a protective coating, and a polymer extended release [ER] sports injuries are excellent candidates relaxants? membrane that controls the rate of hydrochloride) is the most widely for Myprocam. Skeletal muscle relaxants (SMRs) are drug release. prescribed skeletal muscle a group of structurally unrelated drugs, The formulation of the drug relaxant in the US, is now available 4. What are the common as shown in Figure 1. These are divided ensures early systemic exposure in SA. Myprocam’s safety and efficacy indications for which Myprocam into two categories: to cyclobenzaprine, with a plasma has been shown in more than 20 clinical is used, and what are the common • Antispasticity agents: Used to concentration at four hours that trials. It was recently launched by causes of these ailments? treat muscle spasticity caused by is similar to that observed with Adcock Ingram nationally, making SA Low back pain, neck pain, sports traumatic neurologic injury, multiple cyclobenzaprine IR. the second country (only to the US) to injuries, muscle sprains and strains. sclerosis, and other conditions. However, in contrast to the bring the product to market. Common causes of these conditions • Antispasmodic agents: Used fluctuating peaks and troughs in Myprocam, indicated for the relief of include sports injuries, car accidents, to treat muscular pain or spasm plasma cyclobenzaprine concentration muscle spasm associated with acute work-related injuries, and everyday associated with acute, nonspecific after administration of the IR and painful musculoskeletal conditions, activities, which account for acute musculoskeletal conditions. -

Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW
Guideline Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW Summary To provide the most up-to-date knowledge and current level of best practice for the treatment of withdrawal from alcohol and other drugs such as heroin, and other opioids, benzodiazepines, cannabis and psychostimulants. Document type Guideline Document number GL2008_011 Publication date 04 July 2008 Author branch Centre for Alcohol and Other Drugs Branch contact (02) 9424 5938 Review date 18 April 2018 Policy manual Not applicable File number 04/2766 Previous reference N/A Status Active Functional group Clinical/Patient Services - Pharmaceutical, Medical Treatment Population Health - Pharmaceutical Applies to Area Health Services/Chief Executive Governed Statutory Health Corporation, Board Governed Statutory Health Corporations, Affiliated Health Organisations, Affiliated Health Organisations - Declared Distributed to Public Health System, Ministry of Health, Public Hospitals Audience All groups of health care workers;particularly prescribers of opioid treatments Secretary, NSW Health Guideline Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/ space space Drug and Alcohol Withdrawal Clinical Practice Guidelines - NSW space Document Number GL2008_011 Publication date 04-Jul-2008 Functional Sub group Clinical/ Patient Services - Pharmaceutical Clinical/ Patient Services - Medical Treatment Population Health - Pharmaceutical -

Understanding Benzodiazephine Use, Abuse, and Detection
Siemens Healthcare Diagnostics, the leading clinical diagnostics company, is committed to providing clinicians with the vital information they need for the accurate diagnosis, treatment and monitoring of patients. Our comprehensive portfolio of performance-driven systems, unmatched menu offering and IT solutions, in conjunction with highly responsive service, is designed to streamline workflow, enhance operational efficiency and support improved patient care. Syva, EMIT, EMIT II, EMIT d.a.u., and all associated marks are trademarks of General Siemens Healthcare Diagnostics Inc. All Drugs other trademarks and brands are the Global Division property of their respective owners. of Abuse Siemens Healthcare Product availability may vary from Diagnostics Inc. country to country and is subject 1717 Deerfield Road to varying regulatory requirements. Deerfield, IL 60015-0778 Please contact your local USA representative for availability. www.siemens.com/diagnostics Siemens Global Headquarters Global Siemens Healthcare Headquarters Siemens AG Understanding Wittelsbacherplatz 2 Siemens AG 80333 Muenchen Healthcare Sector Germany Henkestrasse 127 Benzodiazephine Use, 91052 Erlangen Germany Abuse, and Detection Telephone: +49 9131 84 - 0 www.siemens.com/healthcare www.usa.siemens.com/diagnostics Answers for life. Order No. A91DX-0701526-UC1-4A00 | Printed in USA | © 2009 Siemens Healthcare Diagnostics Inc. Syva has been R1 R2 a leading developer N and manufacturer of AB R3 X N drugs-of-abuse tests R4 for more than 30 years. R2 C Now part of Siemens Healthcare ® Diagnostics, Syva boasts a long and Benzodiazepines have as their basic chemical structure successful track record in drugs-of-abuse a benzene ring fused to a seven-membered diazepine ring. testing, and leads the industry in the All important benzodiazepines contain a 5-aryl substituent ring (ring C) and a 1,4–diazepine ring. -

Risk Based Requirements for Medicines Handling
Risk based requirements for medicines handling Including requirements for Schedule 4 Restricted medicines Contents 1. Introduction 2 2. Summary of roles and responsibilities 3 3. Schedule 4 Restricted medicines 4 4. Medicines acquisition 4 5. Storage of medicines, including control of access to storage 4 5.1. Staff access to medicines storage areas 5 5.2. Storage of S4R medicines 5 5.3. Storage of S4R medicines for medical emergencies 6 5.4. Access to storage for S4R and S8 medicines 6 5.5. Pharmacy Department access, including after hours 7 5.6. After-hours access to S8 medicines in the Pharmacy Department 7 5.7. Storage of nitrous oxide 8 5.8. Management of patients’ own medicines 8 6. Distribution of medicines 9 6.1. Distribution outside Pharmacy Department operating hours 10 6.2. Distribution of S4R and S8 medicines 10 7. Administration of medicines to patients 11 7.1. Self-administration of scheduled medicines by patients 11 7.2. Administration of S8 medicines 11 8. Supply of medicines to patients 12 8.1. Supply of scheduled medicines to patients by health professionals other than pharmacists 12 9. Record keeping 13 9.1. General record keeping requirements for S4R medicines 13 9.2. Management of the distribution and archiving of S8 registers 14 9.3. Inventories of S4R medicines 14 9.4. Inventories of S8 medicines 15 10. Destruction and discards of S4R and S8 medicines 15 11. Management of oral liquid S4R and S8 medicines 16 12. Cannabis based products 17 13. Management of opioid pharmacotherapy 18 14. -

Benzodiazepines
Benzodiazepines Using benzodiazepines in Children and Adolescents Overview Benzodiazepines are group of medications used to treat several different conditions. Some examples of these medications include: lorazepam (Ativan®); clonazepam (Rivotril®); alprazolam (Xanax®) and oxazepam (Serax®). Other benzodiazepine medications are available, but are less commonly used in children and adolescents. What are benzodiazepines used for? Benzodiazepines may be used for the following conditions: • anxiety disorders: generalized anxiety disorder; social anxiety disorder; post-traumatic stress disorder (PTSD); panic attacks/disorder; excessive anxiety prior to surgery • sleep disorders: trouble sleeping (insomnia); waking up suddenly with great fear (night terrors); sleepwalking • seizure disorders (epilepsy) • alcohol withdrawal • treatment of periods of extreme slowing or excessive purposeless motor activity (catatonia) Your doctor may be using this medication for another reason. If you are unclear why this medication is being prescribed, please ask your doctor. How do benzodiazepines work? Benzodiazepines works by affecting the activity of the brain chemical (neurotransmitter) called GABA. By enhancing the action of GABA, benzodiazepines have a calming effect on parts of the brain that are too excitable. This in turn helps to manage anxiety, insomnia, and seizure disorders. How well do benzodiazepines work in children and adolescents? When used to treat anxiety disorders, benzodiazepines decrease symptoms such as nervousness, fear, and excessive worrying. Benzodiazepines may also help with the physical symptoms of anxiety, including fast or strong heart beat, trouble breathing, dizziness, shakiness, sweating, and restlessness. Typically, benzodiazepines are prescribed to manage anxiety symptoms that are uncomfortable, frightening or interfere with daily activities for a short period of time before conventional anti-anxiety treatments like cognitive-behavioural therapy or anti-anxiety takes effect. -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Medications in Long-Term Care: When Less Is More
Medications in Long-Term Care: When Less is More a, b,c Thomas W. Meeks, MD *, John W. Culberson, MD , d,e Monica S. Horton, MD, MSc KEYWORDS Long-term care Inappropriate prescribing Polypharmacy Psychotropics Opiates Sedatives HISTORY OF MEDICATION REDUCTION IN LONG-TERM CARE The Nursing Home Reform Act (OBRA-87) enacted in 1987 called for sweeping changes in the standards of care in nursing homes in accordance with new, more demanding federal regulations. For example, OBRA-87 called for a new approach to the use of antipsychotics in persons with dementia. Because antipsychotics were regarded as frequently inappropriate chemical restraints in long-term care (LTC), OBRA-87 mandated dose reductions in antipsychotics in an effort to discontinue them whenever possible. OBRA-87 proposed that a safer, more supportive environ- ment in LTC settings would facilitate such reductions in antipsychotic doses.1 Overall rates of potentially inappropriate prescribing in older adults have ranged from 12% to 40%, depending on the setting, criteria, and population sampled.2 Devel- oping from burgeoning concerns for polypharmacy and potential iatrogenic toxicity of medication in older adults, expert consensus lists of potentially inappropriate pharma- cotherapy in the elderly (PIPE) began to emerge. In general, drugs with activity in the central nervous system (CNS) were commonly placed on these PIPE lists, and hence our focus on such medications in this review. This work was supported by the following Geriatric Academic Career Awards: (1) K01 HP00047-02 (Dr. Meeks) (2) K01 HP00080-02 (Dr. Culberson) (3) K01 HP00114-02 (Dr. Horton). The authors have no conflicts of interest to disclose. -

Calculating Equivalent Doses of Oral Benzodiazepines
Calculating equivalent doses of oral benzodiazepines Background Benzodiazepines are the most commonly used anxiolytics and hypnotics (1). There are major differences in potency between different benzodiazepines and this difference in potency is important when switching from one benzodiazepine to another (2). Benzodiazepines also differ markedly in the speed in which they are metabolised and eliminated. With repeated daily dosing accumulation occurs and high concentrations can build up in the body (mainly in fatty tissues) (2). The degree of sedation that they induce also varies, making it difficult to determine exact equivalents (3). Answer Advice on benzodiazepine conversion NB: Before using Table 1, read the notes below and the Limitations statement at the end of this document. Switching benzodiazepines may be advantageous for a variety of reasons, e.g. to a drug with a different half-life pre-discontinuation (4) or in the event of non-availability of a specific benzodiazepine. With relatively short-acting benzodiazepines such as alprazolam and lorazepam, it is not possible to achieve a smooth decline in blood and tissue concentrations during benzodiazepine withdrawal. These drugs are eliminated fairly rapidly with the result that concentrations fluctuate with peaks and troughs between each dose. It is necessary to take the tablets several times a day and many people experience a "mini-withdrawal", sometimes a craving, between each dose. For people withdrawing from these potent, short-acting drugs it has been advised that they switch to an equivalent dose of a benzodiazepine with a long half life such as diazepam (5). Diazepam is available as 2mg tablets which can be halved to give 1mg doses. -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

Drugs of Abuse: Benzodiazepines
Drugs of Abuse: Benzodiazepines What are Benzodiazepines? Benzodiazepines are central nervous system depressants that produce sedation, induce sleep, relieve anxiety and muscle spasms, and prevent seizures. What is their origin? Benzodiazepines are only legally available through prescription. Many abusers maintain their drug supply by getting prescriptions from several doctors, forging prescriptions, or buying them illicitly. Alprazolam and diazepam are the two most frequently encountered benzodiazepines on the illicit market. Benzodiazepines are What are common street names? depressants legally available Common street names include Benzos and Downers. through prescription. Abuse is associated with What do they look like? adolescents and young The most common benzodiazepines are the prescription drugs ® ® ® ® ® adults who take the drug Valium , Xanax , Halcion , Ativan , and Klonopin . Tolerance can orally or crush it up and develop, although at variable rates and to different degrees. short it to get high. Shorter-acting benzodiazepines used to manage insomnia include estazolam (ProSom®), flurazepam (Dalmane®), temazepam (Restoril®), Benzodiazepines slow down and triazolam (Halcion®). Midazolam (Versed®), a short-acting the central nervous system. benzodiazepine, is utilized for sedation, anxiety, and amnesia in critical Overdose effects include care settings and prior to anesthesia. It is available in the United States shallow respiration, clammy as an injectable preparation and as a syrup (primarily for pediatric skin, dilated pupils, weak patients). and rapid pulse, coma, and possible death. Benzodiazepines with a longer duration of action are utilized to treat insomnia in patients with daytime anxiety. These benzodiazepines include alprazolam (Xanax®), chlordiazepoxide (Librium®), clorazepate (Tranxene®), diazepam (Valium®), halazepam (Paxipam®), lorzepam (Ativan®), oxazepam (Serax®), prazepam (Centrax®), and quazepam (Doral®). -

CYCLOBENZAPRINE (Brand Name: Flexeril®, Amrix®)
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section CYCLOBENZAPRINE ® ® (Brand Name: Flexeril , Amrix ) March 2020 Introduction: Illicit Uses: Cyclobenzaprine is a central nervous system Anecdotal reports found on the Internet suggest (CNS) muscle relaxant intended for short-term use that individuals are taking cyclobenzaprine alone or in the treatment of pain, tenderness and limitation of in combination with other illicit drugs to produce or motion caused by muscle spasms. Cyclobenzaprine enhance psychoactive effects. Individuals have may enhance the effects of other CNS depressants reported taking cyclobenzadrine both orally and including alcohol, barbiturates, benzodiazepines intra-nasally at doses ranging from 10 mg to 60 mg. and narcotics and anecdotal reports indicate it is Sedation, relaxation and increased heart rate were used non-medically to induce euphoria and the most common effects reported. Euphoria was relaxation. reported by a smaller number of individuals. Licit Uses: Illicit Distribution: Cyclobenzaprine hydrochloride is approved for Several indicators suggest that cyclobenzaprine use in the United States as a muscle relaxant. It is is being intentionally misused or abused. According marketed under the brand names Flexeril® and to the American Association of Poison Control Amrix® and as generic formulations in 5, 7.5, and 10 Centers, 10,615 case mentions and 4,444 single mg tablets intended for short-term (2 to 3 week) oral exposures were associated with cyclobenzaprine in administration. The usual starting dose is 5 mg, 2016, resulting in 75 major medical outcomes and three times per day. The maximum recommended four deaths among single substance exposures. In dose is 10 mg, three times daily. -
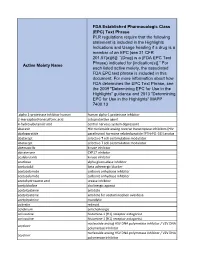
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha.