Top-Down Modulation of Sensory Cortex Gates Perceptual Learning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

Interoception: the Sense of the Physiological Condition of the Body AD (Bud) Craig
500 Interoception: the sense of the physiological condition of the body AD (Bud) Craig Converging evidence indicates that primates have a distinct the body share. Recent findings that compel a conceptual cortical image of homeostatic afferent activity that reflects all shift resolve these issues by showing that all feelings from aspects of the physiological condition of all tissues of the body. the body are represented in a phylogenetically new system This interoceptive system, associated with autonomic motor in primates. This system has evolved from the afferent control, is distinct from the exteroceptive system (cutaneous limb of the evolutionarily ancient, hierarchical homeostatic mechanoreception and proprioception) that guides somatic system that maintains the integrity of the body. These motor activity. The primary interoceptive representation in the feelings represent a sense of the physiological condition of dorsal posterior insula engenders distinct highly resolved the entire body, redefining the category ‘interoception’. feelings from the body that include pain, temperature, itch, The present article summarizes this new view; more sensual touch, muscular and visceral sensations, vasomotor detailed reviews are available elsewhere [1,2]. activity, hunger, thirst, and ‘air hunger’. In humans, a meta- representation of the primary interoceptive activity is A homeostatic afferent pathway engendered in the right anterior insula, which seems to provide Anatomical characteristics the basis for the subjective image of the material self as a feeling Cannon [3] recognized that the neural processes (auto- (sentient) entity, that is, emotional awareness. nomic, neuroendocrine and behavioral) that maintain opti- mal physiological balance in the body, or homeostasis, must Addresses receive afferent inputs that report the condition of the Atkinson Pain Research Laboratory, Division of Neurosurgery, tissues of the body. -

The Contribution of Sensory System Functional Connectivity Reduction to Clinical Pain in Fibromyalgia
1 The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia Jesus Pujol1,2, Dídac Macià1, Alba Garcia-Fontanals3, Laura Blanco-Hinojo1,4, Marina López-Solà1,5, Susana Garcia-Blanco6, Violant Poca-Dias6, Ben J Harrison7, Oren Contreras-Rodríguez1, Jordi Monfort8, Ferran Garcia-Fructuoso6, Joan Deus1,3 1MRI Research Unit, CRC Mar, Hospital del Mar, Barcelona, Spain. 2Centro Investigación Biomédica en Red de Salud Mental, CIBERSAM G21, Barcelona, Spain. 3Department of Clinical and Health Psychology, Autonomous University of Barcelona, Spain. 4Human Pharmacology and Neurosciences, Institute of Neuropsychiatry and Addiction, Hospital del Mar Research Institute, Barcelona, Spain. 5Department of Psychology and Neuroscience. University of Colorado, Boulder, Colorado. 6Rheumatology Department, Hospital CIMA Sanitas, Barcelona, Spain. 7Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne, Melbourne, Australia. 8Rheumatology Department, Hospital del Mar, Barcelona, Spain The submission contains: The main text file (double-spaced 39 pages) Six Figures One supplemental information file Corresponding author: Dr. Jesus Pujol Department of Magnetic Resonance, CRC-Mar, Hospital del Mar Passeig Marítim 25-29. 08003, Barcelona, Spain Email: [email protected] Telephone: + 34 93 221 21 80 Fax: + 34 93 221 21 81 Synopsis/ Summary Clinical pain in fibromyalgia is associated with functional changes at different brain levels in a pattern suggesting a general weakening of sensory integration. 2 Abstract Fibromyalgia typically presents with spontaneous body pain with no apparent cause and is considered pathophysiologically to be a functional disorder of somatosensory processing. We have investigated potential associations between the degree of self-reported clinical pain and resting-state brain functional connectivity at different levels of putative somatosensory integration. -
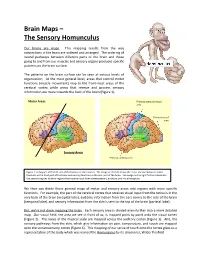
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Cortical and Subcortical Mechanisms for Sound Processing Jennifer Blackwell University of Pennsylvania, [email protected]
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2019 Cortical And Subcortical Mechanisms For Sound Processing Jennifer Blackwell University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Neuroscience and Neurobiology Commons Recommended Citation Blackwell, Jennifer, "Cortical And Subcortical Mechanisms For Sound Processing" (2019). Publicly Accessible Penn Dissertations. 3275. https://repository.upenn.edu/edissertations/3275 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/3275 For more information, please contact [email protected]. Cortical And Subcortical Mechanisms For Sound Processing Abstract The uda itory cortex is essential for encoding complex and behaviorally relevant sounds. Many questions remain concerning whether and how distinct cortical neuronal subtypes shape and encode both simple and complex sound properties. In chapter 2, we tested how neurons in the auditory cortex encode water-like sounds perceived as natural by human listeners, but that we could precisely parametrize. The ts imuli exhibit scale-invariant statistics, specifically temporal modulation within spectral bands scaled with the center frequency of the band. We used chronically implanted tetrodes to record neuronal spiking in rat primary auditory cortex during exposure to our custom stimuli at different rates and cycle-decay constants. We found that, although neurons exhibited selectivity for subsets of stimuli with specific ts atistics, over the population responses were stable. These results contribute to our understanding of how auditory cortex processes natural sound statistics. In chapter 3, we review studies examining the role of different cortical inhibitory interneurons in shaping sound responses in auditory cortex. We identify the findings that support each other and the mechanisms that remain unexplored. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

Role of Primary Somatic Sensory Cortex in the Categorization of Tactile Stimuli: Effects of Lesions
Exp Brain Res (1997) 115:357–360 © Springer-Verlag 1997 RESEARCH NOTE &roles:Antonio Zainos · Hugo Merchant · Adrián Hernández Emilio Salinas · Ranulfo Romo Role of primary somatic sensory cortex in the categorization of tactile stimuli: effects of lesions &misc:Received: 28 October 1996 / Accepted: 27 January 1997 &p.1:Abstract We lesioned the right primary somatic sensory stimulus speeds irrespective of the particular finger stim- (SI) cortex in two monkeys trained to categorize the ulated, the distance traversed by the probe, and the stim- speed of moving tactile stimuli. Animals performed the ulus direction. Therefore, we found this paradigm well task by pressing with the right hand one of two target suited for investigating, in somesthetic and motor corti- switches to indicate whether the speed of a probe mov- cal areas, the neuronal processes associated with the ani- ing across the glabrous skin of the left hand was low or mal’s performance and with the categorization process. high. Sensory performance was evaluated with psycho- Using the tactile categorization task, we previously re- metric techniques and motor behavior was monitored by corded the responses of neurons in primary somatic sen- measuring the reaction (RT) and movement (MT) times sory (SI) cortex with receptive fields on the finger tips before the experiment and throughout the 60 days after (Romo et al. 1996). We found a class of neurons whose the ablation of SI cortex. After the lesion, there was a discharge rates varied smoothly with stimulus speed. slight increase in the RTs but no change in the MTs, in- However, these neuronal responses were also present dicating that removal of SI cortex did not affect the ani- when the same stimuli were delivered passively and, fur- mals’ capacity to detect the stimuli. -

Within-Finger Maps of Tactile and Nociceptive Input in the Human Parietal Cortex 2 3 Flavia Mancini1,2*, Martin I
bioRxiv preprint doi: https://doi.org/10.1101/599167; this version posted April 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Within-finger maps of tactile and nociceptive input in the human parietal cortex 2 3 Flavia Mancini1,2*, Martin I. Sereno3, Min-Ho Lee4, Giandomenico D Iannetti2, Irene Tracey4 4 5 1Computational and Biological Learning, Department of Engineering, University of 6 Cambridge, Cambridge, UK 7 2Department of Neuroscience, Physiology and Pharmacology, University College London, 8 London, UK 9 3Department of Psychology, San Diego State University, San Diego, USA 10 4Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical 11 Neurosciences, University of Oxford, Oxford, UK 12 13 14 15 *Corresponding author: 16 Dr Flavia Mancini 17 Computational and Biological Learning, Department of Engineering, University of Cambridge 18 Trumpington Street, Cambridge, CB2 1PZ 19 [email protected] 20 21 22 23 bioRxiv preprint doi: https://doi.org/10.1101/599167; this version posted April 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 24 Abstract 25 26 The spatial representation of nociceptive input in the human parietal cortex is not fully 27 understood. For instance, it is known that the primary somatosensory cortex (S1) contains a 28 representation of nociceptive-selective input to different fingers, but it is unclear whether S1 29 subdivisions contain finer-grained, within-finger maps of nociceptive input. -
Cerebral Cortex
Cerebral Cortex • Brain’s most complex area with billions of neurons and trillions of synapses: the tissue responsible for mental activities: consciousness, perceives sensations, skilled movements, emotional awareness, memory, thinking, language ability, motivation 1 John Lorber, Science 210:1232 (1980) “There a young student at this university who has an IQ of 126, has gained a first-class honors degree in mathematics, and is socially completely normal. And yet the boy has virtually no brain.” “The student’s physician at the university noticed that the student had slightly larger than normal head… When we did a brain scan on him we saw that instead of the normal 4.5 cm thickness of brain tissue there was just a millimeter or so. His cranium is filled with CSF.” How is this possible? What does it tell us? Do you think this would be OK if it happened to an adult? To a 15 year old? To a 5 year old? To a neonate? 3 Types of Cerebral Cortex • Neocortex – Newest in evolution – About 90% of total – 6 layers, most complex • Paleocortex – Associated with olfactory system, the parahippocampal gyrus, uncus – fewer than 6 layers • Archicortex – Hippocampal formation; limbic system – 3 layers, most primitive • Mesocortex – Cingulate gyrus, insular cortex – Transitional between archicortex and neocortex 5 The perks of having a neocortex • The words used to describe the higher mental capacities of animals with a large neocortex include: – CONSCIOUSNESS – FREE WILL – INTELLIGENCE – INSIGHT • Animals with much simpler brains learn well, so LEARNING should not be among these capacities (Macphail 1982). • A species could have genetically determined mechanisms, acquired through evolutionary selection, for taking advantage of the regular features of the environment, or they could have learned through direct experience. -

How Does the Brain Produce Movement?
p CHAPTER 10 How Does the Brain Produce Movement? The Hierarchical Control The Basal Ganglia and of Movement the Cerebellum The Forebrain and Movement Initiation The Basal Ganglia and Movement Force The Brainstem and Species-Typical Movement Focus on Disorders: Tourette’s Syndrome Focus on Disorders: Autism The Cerebellum and Movement Skill The Spinal Cord and Movement Execution Focus on Disorders: Paraplegia The Organization of the Somatosensory System The Organization of the Somatosensory Receptors and Sensory Perception Motor System Dorsal-Root Ganglion Neurons The Motor Cortex The Somatosensory Pathways to the Brain The Corticospinal Tracts Spinal-Cord Responses to Somatosensory Input The Motor Neurons The Vestibular System and Balance The Control of Muscles Exploring the Somatosensory The Motor Cortex and Skilled System Movements The Somatosensory Homunculus Investigating Neural Control of Skilled Movements The Effects of Damage to the Somatosensory Cortex The Control of Skilled Movements in Other Species The Somatosensory Cortex and Complex How Motor Cortex Damage Affects Skilled Movement Movements Kevork Djansezian/AP Photo Micrograph: Dr. David Scott/Phototake 354 I p amala is a female Indian elephant that lives at the In one way, however, Kamala uses this versatile trunk zoo in Calgary, Canada. Her trunk, which is really very unusually for an elephant (Onodera & Hicks, 1999). K just a greatly extended upper lip and nose, con- She is one of only a few elephants in the world that paints sists of about 2000 fused muscles. A pair of nostrils runs its with its trunk (Figure 10-1). Like many artists, she paints length and fingerlike projections are located at its tip. -

Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G
Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA How do we move? Decision to move (Executive or Cognitive Stage) Access Movement Motor (Feed-back Program or Muscle (Effector or Stage) CNS Stage) Decision to move (Executive or Cognitive Stage) Sensory feedback Higher brain functions Access Movement Motor (Feed-back Program or Muscle (Effector or Stage) CNS Stage) Spinal Processing Decision to move (Executive or Cognitive Higher brain functions: Sensory feedback: Stage) Motor cortex, limbic Mechanoreceptors, system, thalamus, Nociceptors, et al. cerebellum Access Movement Motor (Feed-back Program or Muscle (Effector or Stage) CNS Stage) Spinal Processing Movement Patterns • Movements are coded throughout the nervous system. • They are not coded in a single place such as the motor cortex, cerebellum, thalamus, or spinal cord. • Movement appears to be a series of learned and activated behaviors. • We learn by making mistakes. • Just as we can learn an adaptive movement pattern, we can learn a maladaptive pattern. • The difference between adaptive and maladaptive is often a matter of timing. Organization of CNS • Organized into relatively autonomous functional systems, for example: • Discrete systems for 5 special senses • Different classes for movement, such as: • Eye, hand, upper extremity, lower extremity • Language • But all of must function cooperatively together. Transformation of sensory information • Information is either attenuated or amplified at every synaptic relay, depending on the state of arousal. • Nearly all sensory information is organized topographically, at each level. • This forms a sensory map of information from the receptor surface. • This is not just represented spatially, but also by density of receptors.