Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

A Revised Computational Neuroanatomy for Motor Control
This is the author’s final version; this article has been accepted for publication in the Journal of Cognitive Neuroscience 1 A Revised Computational Neuroanatomy for Motor Control 2 Shlomi Haar1, Opher Donchin2,3 3 1. Department of BioEngineering, Imperial College London, UK 4 2. Department of Biomedical Engineering, Ben-Gurion University of the Negev, Israel 5 3. Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Israel 6 7 Corresponding author: Shlomi Haar ([email protected]) 8 Imperial College London, London, SW7 2AZ, UK 9 10 Acknowledgements: We would like to thank Ilan Dinstein, Liad Mudrik, Daniel Glaser, Alex Gail, and 11 Reza Shadmehr for helpful discussions about the manuscript. Shlomi Haar is supported by the Royal 12 Society – Kohn International Fellowship (NF170650). Work on this review was partially supported by 13 DFG grant TI-239/16-1. 14 15 Abstract 16 We discuss a new framework for understanding the structure of motor control. Our approach 17 integrates existing models of motor control with the reality of hierarchical cortical processing and the 18 parallel segregated loops that characterize cortical-subcortical connections. We also incorporate the recent 19 claim that cortex functions via predictive representation and optimal information utilization. Our 20 framework assumes each cortical area engaged in motor control generates a predictive model of a different 21 aspect of motor behavior. In maintaining these predictive models, each area interacts with a different part 22 of the cerebellum and basal ganglia. These subcortical areas are thus engaged in domain appropriate 23 system identification and optimization. This refocuses the question of division of function among different 24 cortical areas. -

Motor Control: a Sense of Movement
RESEARCH HIGHLIGHTS MOTOR CONTROL A sense of movement Neuroscience textbooks tell us that with a shorter latency after S1 stimu- whisker protraction, and that M1retract the motor cortex controls move- lation than after M1 stimulation. only induces whisker retraction ment. But now, Carl Petersen and Thus, S1 and M1 are both involved in indirectly, through S1 activation. colleagues show that sensory cortex whisker motor and sensory process- By mapping the neural pathways may have an equally important role ing. The M1 area that induced C2 involved in C2 whisker retraction in motor control. retraction was termed M1retract; a and protraction, the authors found The authors showed that a single, more medial M1 region that induced that M1C2 and S1C2 have reciprocal brief deflection of the C2 whisker C2 protraction under microstimula- connections and project to adjacent induced activity in the correspond- tion was termed M1protract. regions in subcortical areas. In the ing barrel column of the primary The authors next investigated the brain stem, this includes the reticular somatosensory cortex (S1), followed functional relevance of this finding. formation as a projection area of M1, by a response in a small area in the By attaching metal particles to the C2 and the spinal trigeminal nuclei as primary motor cortex (M1). Direct whisker and applying a pulsed mag- a projection area of S1. Both areas microstimulation or optogenetic netic field, the authors could evoke project to the facial nucleus, which stimulation of either area induced C2 whisker deflections. The whisker contains whisker motor neurons. a brief retraction of the C2 whisker, retracted in response to this stimulus, Indeed, direct electrical stimula- and this response was abolished when tion of the reticular formation and S1 was inactivated — but not spinal trigeminal nuclei induced when M1 was inactivated — with whisker protraction and retraction, tetrodoxin (TTX). -

Motor Control in the Brain
Motor Control in the Brain Travis DeWolf School of Computer Science University of Waterloo Waterloo, ON Canada December 19, 2008 Abstract There has been much progress in the development of a model of motor control in the brain in the last decade; from the improved method for mathematically extracting the predicted movement direction from a population of neurons to the application of optimal control theory to motor control models, much work has been done to further our understanding of this area. In this paper recent literature is reviewed and the direction of future research is examined. 1 Introduction In the last decade there has been a push from investigating the coordinate system used in the brain, which has been the focus of research since the 1970s, back toward using reductionist experiment techniques and developing more encompassing models of motor control in the brain[28]. Optimal control theory applied to motor control in the brain has also recently started being explored as a framework for models. This has lead to research into the underlying biology of the implementations of internal models of movement and reinforcement learning reward systems in the brain[32]. In addition to this, with the discovery of motor primitives[24], which are often referred to as the `building blocks of movement', many of the ideas about neural activity carried out in the motor cortex need be reworked. The analysis techniques employed by researchers have been improved and of course the computing power available has increased enormously, and these developments have in turn opened the door to the need for further mathematical technique developments for accurate movement analysis and prediction. -

Interoception: the Sense of the Physiological Condition of the Body AD (Bud) Craig
500 Interoception: the sense of the physiological condition of the body AD (Bud) Craig Converging evidence indicates that primates have a distinct the body share. Recent findings that compel a conceptual cortical image of homeostatic afferent activity that reflects all shift resolve these issues by showing that all feelings from aspects of the physiological condition of all tissues of the body. the body are represented in a phylogenetically new system This interoceptive system, associated with autonomic motor in primates. This system has evolved from the afferent control, is distinct from the exteroceptive system (cutaneous limb of the evolutionarily ancient, hierarchical homeostatic mechanoreception and proprioception) that guides somatic system that maintains the integrity of the body. These motor activity. The primary interoceptive representation in the feelings represent a sense of the physiological condition of dorsal posterior insula engenders distinct highly resolved the entire body, redefining the category ‘interoception’. feelings from the body that include pain, temperature, itch, The present article summarizes this new view; more sensual touch, muscular and visceral sensations, vasomotor detailed reviews are available elsewhere [1,2]. activity, hunger, thirst, and ‘air hunger’. In humans, a meta- representation of the primary interoceptive activity is A homeostatic afferent pathway engendered in the right anterior insula, which seems to provide Anatomical characteristics the basis for the subjective image of the material self as a feeling Cannon [3] recognized that the neural processes (auto- (sentient) entity, that is, emotional awareness. nomic, neuroendocrine and behavioral) that maintain opti- mal physiological balance in the body, or homeostasis, must Addresses receive afferent inputs that report the condition of the Atkinson Pain Research Laboratory, Division of Neurosurgery, tissues of the body. -

The Contribution of Sensory System Functional Connectivity Reduction to Clinical Pain in Fibromyalgia
1 The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia Jesus Pujol1,2, Dídac Macià1, Alba Garcia-Fontanals3, Laura Blanco-Hinojo1,4, Marina López-Solà1,5, Susana Garcia-Blanco6, Violant Poca-Dias6, Ben J Harrison7, Oren Contreras-Rodríguez1, Jordi Monfort8, Ferran Garcia-Fructuoso6, Joan Deus1,3 1MRI Research Unit, CRC Mar, Hospital del Mar, Barcelona, Spain. 2Centro Investigación Biomédica en Red de Salud Mental, CIBERSAM G21, Barcelona, Spain. 3Department of Clinical and Health Psychology, Autonomous University of Barcelona, Spain. 4Human Pharmacology and Neurosciences, Institute of Neuropsychiatry and Addiction, Hospital del Mar Research Institute, Barcelona, Spain. 5Department of Psychology and Neuroscience. University of Colorado, Boulder, Colorado. 6Rheumatology Department, Hospital CIMA Sanitas, Barcelona, Spain. 7Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne, Melbourne, Australia. 8Rheumatology Department, Hospital del Mar, Barcelona, Spain The submission contains: The main text file (double-spaced 39 pages) Six Figures One supplemental information file Corresponding author: Dr. Jesus Pujol Department of Magnetic Resonance, CRC-Mar, Hospital del Mar Passeig Marítim 25-29. 08003, Barcelona, Spain Email: [email protected] Telephone: + 34 93 221 21 80 Fax: + 34 93 221 21 81 Synopsis/ Summary Clinical pain in fibromyalgia is associated with functional changes at different brain levels in a pattern suggesting a general weakening of sensory integration. 2 Abstract Fibromyalgia typically presents with spontaneous body pain with no apparent cause and is considered pathophysiologically to be a functional disorder of somatosensory processing. We have investigated potential associations between the degree of self-reported clinical pain and resting-state brain functional connectivity at different levels of putative somatosensory integration. -
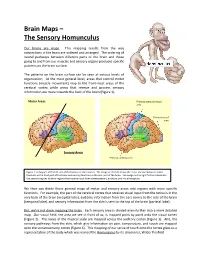
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Sensory Change Following Motor Learning
A. M. Green, C. E. Chapman, J. F. Kalaska and F. Lepore (Eds.) Progress in Brain Research, Vol. 191 ISSN: 0079-6123 Copyright Ó 2011 Elsevier B.V. All rights reserved. CHAPTER 2 Sensory change following motor learning { k { { Andrew A. G. Mattar , Sazzad M. Nasir , Mohammad Darainy , and { } David J. Ostry , ,* { Department of Psychology, McGill University, Montréal, Québec, Canada { Shahed University, Tehran, Iran } Haskins Laboratories, New Haven, Connecticut, USA k The Roxelyn and Richard Pepper Department of Communication Sciences and Disorders, Northwestern University, Evanston, Illinois, USA Abstract: Here we describe two studies linking perceptual change with motor learning. In the first, we document persistent changes in somatosensory perception that occur following force field learning. Subjects learned to control a robotic device that applied forces to the hand during arm movements. This led to a change in the sensed position of the limb that lasted at least 24 h. Control experiments revealed that the sensory change depended on motor learning. In the second study, we describe changes in the perception of speech sounds that occur following speech motor learning. Subjects adapted control of speech movements to compensate for loads applied to the jaw by a robot. Perception of speech sounds was measured before and after motor learning. Adapted subjects showed a consistent shift in perception. In contrast, no consistent shift was seen in control subjects and subjects that did not adapt to the load. These studies suggest that motor learning changes both sensory and motor function. Keywords: motor learning; sensory plasticity; arm movements; proprioception; speech motor control; auditory perception. Introduction the human motor system and, likewise, to skill acquisition in the adult nervous system. -

Motor Control Paper“ for Our Common Script - IPNFA - Nicola Fischer, Carsten Schaefer „Well-Fed-Version“
suggestion for a „Motor Control Paper“ for our common script - IPNFA - Nicola Fischer, Carsten Schaefer „well-fed-version“ _______________________________________ Motor Control _______________________________________ 1. Definition and Contributions of Motor Control 2. Postural control 3. Activities 1. Definitions and Contributions Ø Definition of Motor Control “Motor control is defined as the ability to regulate or direct the mechanisms essential to movement.” (Shumway-Cook & Woollacott 2011, p.4; Horak et al 1997) Relevant questions are: - “How does the central nervous system (CNS) organize the many individual muscles and joints into coordinated functional movements? (Bernstein 1967; Shumway-Cook & Woollacott 2011; Magill 2003; Schmidt & Lee 2011) - How is sensory information from the environment and the body used to select and control movement? - What is the best way to study movement, and how can movement problems be quantified in patients with motor control problems?” (Shumway-Cook & Woollacott 2011, p.4) “Movement emerges from interactions between the individual, the task and the environment.” (Shumway-Cook & Woollacott 2011, p.5) figure 1: adapted from Shumway-Cook (Shumway-Cook & Woollacott 2011) Ø Theories of Motor Control § Reflex Theory e.g. Sir. Ch. Sherrington (Sherrington 1947) § Hierarchical Theory e.g. Rudolf Magnus, Arnold Gesell § Motor Programming Theory e.g. Karl Lashley (Fitch & Martins 2014) Nicolai Bernstein … as a base for the Systems Theory (Bernstein 1967) § Systems Theory / Dynamic Action Theory / Dynamic Pattern Theory - self organizing systems e.g. J.A. Scott Kelso, Viktor Jirsa (Jirsa & Kelso 2004) § Ecological Theory e.g. James Gibson (Gibson 1983) Ø Sensory Contributions to Motor Control For the closed-loop control system, sensory (or afferent) information is necessary to regulate our suggestion for a „Motor Control Paper“ for our common script - IPNFA - Nicola Fischer, Carsten Schaefer „well-fed-version“ movements (Adams 1971; Schmidt & Lee 2011). -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

The Perception for Action Control Theory (PACT): a Perceptuo-Motor Theory of Speech Perception Jean-Luc Schwartz, Anahita Basirat, Lucie Ménard, Marc Sato
The Perception for Action Control Theory (PACT): a perceptuo-motor theory of speech perception Jean-Luc Schwartz, Anahita Basirat, Lucie Ménard, Marc Sato To cite this version: Jean-Luc Schwartz, Anahita Basirat, Lucie Ménard, Marc Sato. The Perception for Action Control Theory (PACT): a perceptuo-motor theory of speech perception. Journal of Neurolinguistics, Elsevier, 2012, 25 (5), pp.336-354. 10.1016/j.jneuroling.2009.12.004. hal-00442367 HAL Id: hal-00442367 https://hal.archives-ouvertes.fr/hal-00442367 Submitted on 21 Dec 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The Perception for Action Control Theory (PACT): a perceptuo-motor theory of speech perception Jean-Luc Schwartz (1), Anahita Basirat (1), Lucie Ménard (2), Marc Sato (1) (1) GIPSA-Lab, Speech and Cognition Department (ICP), UMR 5216 CNRS – Grenoble University, France (2) Laboratoire de Phonétique, UQAM / CRLMB, Montreal, Canada Abstract It is an old-standing debate in the field of speech communication to determine whether speech perception involves auditory or multisensory representations and processing, independently on any procedural knowledge about the production of speech units or on the contrary if it is based on a recoding of the sensory input in terms of articulatory gestures, as posited in the Motor Theory of Speech Perception. -

Proprioception in Motor Learning: Lessons from a Deafferented Subject
Exp Brain Res DOI 10.1007/s00221-015-4315-8 RESEARCH ARTICLE Proprioception in motor learning: lessons from a deafferented subject N. Yousif1 · J. Cole2 · J. Rothwell3 · J. Diedrichsen4 Received: 11 July 2014 / Accepted: 7 May 2015 © Springer-Verlag Berlin Heidelberg 2015 Abstract Proprioceptive information arises from a vari- movement in the perturbed direction. This suggests that ety of channels, including muscle, tendon, and skin affer- this form of learning may depend on static position sense at ents. It tells us where our static limbs are in space and the end of the movement. Our results indicate that dynamic how they are moving. It remains unclear however, how and static proprioception play dissociable roles in motor these proprioceptive modes contribute to motor learning. learning. Here, we studied a subject (IW) who has lost large myeli- nated fibres below the neck and found that he was strongly Keywords Proprioception · Motor control · impaired in sensing the static position of his upper limbs, Deafferentation · Force field learning when passively moved to an unseen location. When making reaching movements however, his ability to discriminate in which direction the trajectory had been diverted was unim- Introduction paired. This dissociation allowed us to test the involve- ment of static and dynamic proprioception in motor learn- Proprioception is a collective term that refers to non-visual ing. We found that IW showed a preserved ability to adapt input that tells us where our body is in space. Propriocep- to force fields when visual feedback was present. He was tion has an important function in normal motor control even sensitive to the exact form of the force perturbation, and motor learning.