Studies on Seed Fats and Fatty Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Details of Agreement's Executed in Form – III Sl. No Appl. No. Applicant
Details of Agreement’s Executed in Form – III Sl. Appl. Applicant Name & Contact For Name of the Project / Bioresources Agreement Patent No No. Details m Invention signed on No 1 100 M/s. Scitech Centre, III Invention relates to 1.Glycyrrhiza glabra 12.06.2007 7, Prabhat Nagar, composition and a process 2.Asparagus officinalis Jogeshwari (West), for preparation of a 3.Angelice officinalis Mumbai – 400 012, composition for 4.pimpinella anisum Maharashtra, manufacturing textured 5.Azdiracta indica 6.Acacia India. soluble container using catechu 7.Acorus calamus herbal texturing agent 8.Andrographis paniculata 9.Berberis asiatica 10.Bergenia cordifolia 11.Boerhaavia diffusa 12.Curcuma longa 13.Cuminum cyminum 14.Cinnamomum zilanicum 15.Coriandrum sativum 16.Centella asiatica 17. Clerodendrum paniculatum 18. Dioscorea bulbifera 19.Echinecea purpurea 20.Eclipta alba 21.Foeniculum vulgare 22.Gingiber officinale 23.Gymnema salvastre 24.hemidesmus indicus 25.Hydrastis urge or 26.Nardostachy jatamansi 27.Pueraria tuberose 28.Phyllanthus amarus 29.Picorrhiza kurroa 30.Pluchea lanceolata 31.Ricinus communis 32.Rauvolfia indica 33.Rubia cordifolia 34.Sida cordifolia 35.Saraca asoca 36.Saussurea lappa 37.Terminalia chebula 38.Tinospora cordifolia 39.Tylophora indica 40.Valeriana officinalis 41.Withiana somnifera 2 79 M/s. Vasundhara, III Water detoxication by Coconut Coir 21.06.2007 15, Saheed Nagar, using coconut coir Bhubaneswar – 751 007, Orissa, India. 3 80 M/s. Vasundhara, III Water detoxication by Bacha (Acorus calamus) 21.06.2007 15, Saheed Nagar, using bacha (Acorus rhizomes Bhubaneswar – 751 007, calamus) rhizomes extract Orissa, India. 4 81 M/s. Vasundhara, III Water detoxication by Jamun seed (Syzygium cumini 21.06.2007 15, Saheed Nagar, using Syzygium cumini (L.) Skeels) Bhubaneswar – 751 007, seed extract. -

Oilseeds As Crop Biofactories for Industrial Raw Materials
AOF Forum, 2004 Grain & chemical industry drivers Oilseeds as crop ! Global competition increasing biofactories for ! Downward pressure on price and market share industrial raw ! Need to diversify away from commodities ! Need to capture maximum value materials Allan Green ! Desirable to replace petroleum with renewable CSIRO Plant Industry sources of industrial raw materials ! Need for increased biodegradability of C O C O C O industrial products C C C C C C O C O C O C Opportunities for industrial use Seed oils are triglycerides ! Current Australian non-food use of vegetable oils ! Seed oils are comprised almost entirely of is about 12,000 tonnes (~3% of total oil usage) triglycerides composed of fatty acids ! Long-term opportunities exist for:- ! Oils are deposited in the seed in oilbodies - direct use in lubricants and inks - bio-diesel fuels (e.g. rapeseed ME) - specialty oleochemicals - pure fatty acids (e.g. oleic acid) - fatty acid derivatives (e.g. erucamide) - alkyl units for polymers (e.g. nylon) - biodegradable plastics - pharmaceutical proteins Fatty acids are like petrochemicals Oil composition can be changed ! Fatty acids are simply hydrocarbon chains ! Seed oils are needed only for energy of various lengths with a carboxyl group storage and release during germination (~COOH) at one end (i.e. no structural role) ! Fatty acids can have bond types or functional groups that allow them to be ! Fatty acid composition can therefore be cleaved or derivatised by chemical dramatically modified, provided that new processing fatty -

Role of Epoxide Hydrolases in Lipid Metabolism
Biochimie 95 (2013) 91e95 Contents lists available at SciVerse ScienceDirect Biochimie journal homepage: www.elsevier.com/locate/biochi Mini-review Role of epoxide hydrolases in lipid metabolism Christophe Morisseau* Department of Entomology and U.C.D. Comprehensive Cancer Center, One Shields Avenue, University of California, Davis, CA 95616, USA article info abstract Article history: Epoxide hydrolases (EH), enzymes present in all living organisms, transform epoxide-containing lipids to Received 29 March 2012 1,2-diols by the addition of a molecule of water. Many of these oxygenated lipid substrates have potent Accepted 8 June 2012 biological activities: host defense, control of development, regulation of blood pressure, inflammation, Available online 18 June 2012 and pain. In general, the bioactivity of these natural epoxides is significantly reduced upon metabolism to diols. Thus, through the regulation of the titer of lipid epoxides, EHs have important and diverse bio- Keywords: logical roles with profound effects on the physiological state of the host organism. This review will Epoxide hydrolase discuss the biological activity of key lipid epoxides in mammals. In addition, the use of EH specific Epoxy-fatty acids Cholesterol epoxide inhibitors will be highlighted as possible therapeutic disease interventions. Ó Juvenile hormone 2012 Elsevier Masson SAS. All rights reserved. 1. Introduction hydrolyzed by a water molecule [8]. Based on this mechanism, transition-state inhibitors of EHs have been designed (Fig. 1B). Epoxides are three atom cyclic ethers formed by the oxidation of These ureas and amides are tight-binding competitive inhibitors olefins. Because of their highly polarized oxygen-carbon bonds and with low nanomolar dissociation constants (KI) [9] [10]. -

Storage Plans for Value Added MFP Products – Van Dhan
Storage Plans for Value Added MFP Products – Van Dhan Storage Guidelines for MFP and Value Added Products - PMVDY After the processing of Minor Forest Produce (MFP) in Van Dhan Vikas Kendras (VDVKs), the need for storing the value added products becomes imperative as they must be preserved until needed for consumption. This document provides guidelines specifying how locally processed items should be preserved. Proper Storage Requirements: Any given storage system must be easy for maintenance and management. A good storage must be prevented from moisture and excessive air current. A good storage system enables free access in terms of regular check to access the state of the product. Any stored produce must be protected from pests, rodents and birds by allowing proper storage hygiene and maintenance. The storage facility must give ease of loading and offloading as the need arises. This is to create accessibility of the product. Storage Methods: Many people store their produce in the drying place itself which is not the right way of storage. Often the root or leaves are not dried properly which can cause harm if not stored properly. It is best to transfer the clean, dry produce to a cool, moisture-free place where rats and insects cannot follow. Warehouses are large houses or spaces that are commonly used as storage structures. They are especially constructed for the protection of the quantity and quality of processed agricultural products. They ways in which processed items can be stored are as follows: Bag Storage: This is a very popular form of storage. Transportation of the product is done in jute bags, the bags are easy to handle and the jute bag allows you to store different items in the same room. -

3.Eng-Non-Edible Kusum Oil Potential Foliage of Biodiesel Production and Its Productive
IMPACT: International Journal of Research in Engineering & Technology (IMPACT: IJRET) ISSN(P): 2347-4599; ISSN(E): 2321-8843 Vol. 4, Issue 9, Sep 2016, 25-36 © Impact Journals NON-EDIBLE KUSUM OIL: POTENTIAL FOLIAGE OF BIODIESEL PRODUCTION AND ITS PRODUCTIVE USE IN MARINE ENGINES SUDHANSU BHUSAN MOHAPATRA 1, PREMANANDA DAS 2, RAMESH CHANDRA MOHANTY 3 & DHANESWAR SWAIN 4 1Assistant Professor, Centurion University of Technology and Management, Jatni, Bhubaneswar, Odisha, India 2Founder of Research Foundation for Rural and Tribal Resource Development, Bhubaneswar, Odisha, India 3Professor, Centurion University, Odisha, India 4Research Scholar, Centurion University, Odisha India ABSTRACT Biodiesel from non-edible vegetable oils is of paramount significance in India due to insufficient edible oil production. The use of biodiesel has been widely accepted as an effective solution to reduce greenhouse emissions. The high potential of biodiesel in terms of PM, NO x, CO and CO 2 emission reduction may represent an additional motivation for its wide use. However the poor low temperature operability is imperative. According to these observations a different behaviour of the after treatment system, especially as far as control issues of the Diesel Particulate Filter are concerned is also expected. The use of biodiesel as alternative to fossil fuel for light duty CI engines to reduce greenhouse gas emissions was widely investigated. However, poor stability of biodiesel - diesel mixture limits the use of biodiesel to low volume concentrations. This paper presents the results concerning the use of a novel fuel additive package containing, pour-point depressant with the aim to increase the quality and amount of biodiesel in the diesel-biodiesel blends. -

A Art of Essential Oils
The Essence’s of Perfume Materials Glen O. Brechbill FRAGRANCE BOOKS INC. www.perfumerbook.com New Jersey - USA 2009 Fragrance Books Inc. @www.perfumerbook.com GLEN O. BRECHBILL “To my parents & brothers family whose faith in my work & abilities made this manuscript possible” II THE ESSENCES OF PERFUME MATERIALS © This book is a work of non-fiction. No part of the book may be used or reproduced in any manner whatsoever without written permission from the author except in the case of brief quotations embodied in critical articles and reviews. Please note the enclosed book is based on The Art of Fragrance Ingredients ©. Designed by Glen O. Brechbill Library of Congress Brechbill, Glen O. The Essence’s of Perfume Materials / Glen O. Brechbill P. cm. 477 pgs. 1. Fragrance Ingredients Non Fiction. 2. Written odor descriptions to facillitate the understanding of the olfactory language. 1. Essential Oils. 2. Aromas. 3. Chemicals. 4. Classification. 5. Source. 6. Art. 7. Thousand’s of fragrances. 8. Science. 9. Creativity. I. Title. Certificate Registry # 1 - 164126868 Copyright © 2009 by Glen O. Brechbill All Rights Reserved PRINTED IN THE UNITED STATES OF AMERICA 10 9 8 7 6 5 4 3 2 1 First Edition Fragrance Books Inc. @www.perfumerbook.com THE ESSENCE’S OF PERFUME MATERIALS III My book displays the very best of essential oils. It offers a rich palette of natural ingredients and essences. At its fullest it expresses a passion for the art of perfume. With one hundred seventy-seven listings it condenses a great deal of pertinent information in a single text. -

Experimental Investigation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi Method
Advances in Chemical Engineering and Science, 2017, 7, 464-476 http://www.scirp.org/journal/aces ISSN Online: 2160-0406 ISSN Print: 2160-0392 Experimental Investigation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi Method Rabiranjan Murmu1,2, Harekrushna Sutar1*, Sangram Patra1 1Chemical Engineering Department, Indira Gandhi Institute of Technology, Sarang, India. 2Chemical Engineering Department, Indian Institute of Technology, Madras, India How to cite this paper: Murmu, R., Sutar, H. and Patra, S. (2017) Experimental Investi- Abstract gation and Process Optimization of Biodiesel Production from Kusum Oil Using Taguchi The paper focuses on biodiesel production from kusum oil using esterification Method. Advances in Chemical Engineering reaction followed by transesterification reaction in an in-house batch reactor and Science, 7, 464-476. setup. The effects of methanol to oil ratio (M/O), catalyst amount (H SO and https://doi.org/10.4236/aces.2017.74033 2 4 methodoxide) and reaction temperature on acid value and fatty acid methyl Received: September 19, 2017 esters (FAME) is studied. Product has been analysed using FTIR spectroscopy Accepted: October 28, 2017 technique for confirmation of ester group in biodiesel. Experimental data was Published: October 31, 2017 optimized by Taguchi analysis to conclude the optimum variable affecting the Copyright © 2017 by authors and response. In both processes M/O ratio has the significant effect for biodiesel Scientific Research Publishing Inc. production. The obtained biodiesel properties are close to commercial diesel This work is licensed under the Creative fuel and may be rated as an alternative to conventional diesel. The biodiesel Commons Attribution International License (CC BY 4.0). -

Download Product Insert (PDF)
PRODUCT INFORMATION (±)12(13)-EpOME Item No. 52450 Formal Name: (±)12(13)epoxy-9Z-octadecenoic acid Synonyms: (±)2,13-EODE, Isoleukotoxin, COOH (±)-Vernolic Acid MF: C18H32O3 FW: 296.5 Chemical Purity: ≥98% O Supplied as: A solution in methyl acetate NOTE: Relative stereochemistry shown in chemical structure Storage: -20°C Stability: ≥1 year Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures (±)12(13)-EpOME is supplied as a solution in methyl acetate. To change the solvent, simply evaporate the methyl acetate under a gentle stream of nitrogen and immediately add the solvent of choice. Solvents such as ethanol, DMSO, and dimethyl formamide purged with an inert gas can be used. The solubility of (±)12(13)-EpOME in these solvents is approximately 50 mg/ml. Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. If an organic solvent-free solution of (±)12(13)-EpOME is needed, it can be prepared by evaporating the methyl acetate and directly dissolving the neat oil in aqueous buffers. The solubility of (±)12(13)-EpOME in PBS (pH 7.2) is approximately 1 mg/ml. We do not recommend storing the aqueous solution for more than one day. Description (±)12(13)-EpOME is the 12,13-cis epoxide form of linoleic acid (Item Nos. 90150 | 90150.1 | 21909).1,2 It is formed primarily via linoleic acid metabolism by the cytochrome P450 (CYP) isoforms CYP2J2, CYP2C8, and CYP2C9, however, CYP1A1 can contribute to (±)12(13)-EpOME production when pharmacologically induced.2 (±)12(13)-EpOME (500 µM) induces mitochondrial dysfunction and cell death in renal proximal tubule epithelial cells. -

Epomes Act As Immune Suppressors in a Lepidopteran Insect
www.nature.com/scientificreports OPEN EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua Mohammad Vatanparast1, Shabbir Ahmed1, Dong‑Hee Lee2, Sung Hee Hwang3, Bruce Hammock3 & Yonggyun Kim1* Epoxyoctadecamonoenoic acids (EpOMEs) are epoxide derivatives of linoleic acid (9,12‑octadecadienoic acid) and include 9,10‑EpOME and 12,13‑EpOME. They are synthesized by cytochrome P450 monooxygenases (CYPs) and degraded by soluble epoxide hydrolase (sEH). Although EpOMEs are well known to play crucial roles in mediating various physiological processes in mammals, their role is not well understood in insects. This study chemically identifed their presence in insect tissues: 941.8 pg/g of 9,10‑EpOME and 2,198.3 pg/g of 12,13‑EpOME in fat body of a lepidopteran insect, Spodoptera exigua. Injection of 9,10‑EpOME or 12,13‑EpOME into larvae suppressed the cellular immune responses induced by bacterial challenge. EpOME treatment also suppressed the expression of antimicrobial peptide (AMP) genes. Among 139 S. exigua CYPs, an ortholog (SE51385) to human EpOME synthase was predicted and its expression was highly inducible upon bacterial challenge. RNA interference (RNAi) of SE51385 prevented down‑regulation of immune responses at a late stage (> 24 h) following bacterial challenge. A soluble epoxide hydrolase (Se-sEH) of S. exigua was predicted and showed specifc expression in all development stages and in diferent larval tissues. Furthermore, its expression levels were highly enhanced by bacterial challenge in diferent tissues. RNAi reduction of Se‑sEH interfered with hemocyte‑spreading behavior, nodule formation, and AMP expression. To support the immune association of EpOMEs, urea‑based sEH inhibitors were screened to assess their inhibitory activities against cellular and humoral immune responses of S. -

CB-NRM Technical Manual Vol
CB-NRM Technical Manual Vol. 3: Income Generating/Livelihood Development Prepared by The Project for Community-Based Sustainable Natural Resource Management in the Democratic Republic of Timor-Leste Project for Community-Based Sustainable Natural Resource Management in the Democratic Republic of Timor-Leste CB-NRM Technical Manual Vol. 3: Income Generating / Livelihood Development Table of Contents page Chapter 1 Introduction ............................................................................................ 1 1.1 Rationale for the Techniques................................................................................ 1 1.2 Objectives of the Techniques ............................................................................... 1 1.3 Objectives of the Manual ..................................................................................... 1 Chapter 2 Approaches to Effective Transferring of Techniques ......................... 2 2.1 Hands-on Training and Follow-up On-the Job Training (OJT) ........................... 2 2.2 Resource-Based ................................................................................................... 3 2.3 Participatory ......................................................................................................... 3 2.4 Women Centric .................................................................................................... 3 2.5 Continuous Coaching ........................................................................................... 3 2.6 Framework to Transfer -

WRA Species Report
Family: Sapindaceae Taxon: Schleichera oleosa Synonym: Schleichera trijuga Willd. Common Name: Ceylon oak lactree Macassar oiltree Malay lactree Questionaire : current 20090513 Assessor: HPWRA OrgData Designation: EVALUATE Status: Assessor Approved Data Entry Person: HPWRA OrgData WRA Score 1 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 n 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see n Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 n 405 Toxic to animals -
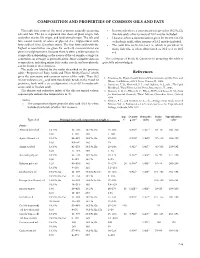
Composition and Properties of Common Oils and Fats References
COMPOSITION AND PROPERTIES OF COMMON OILS AND FaTS This table lists some of the most common naturally occurring • In some oils where a concentration is given for 18:2 9c,12c oils and fats . The list is separated into those of plant origin, fish (linoleic acid), other isomers of 18:2 may be included . and other marine life origin, and land animal origin . The oils and • Likewise, where a concentration is given for 18:3 9c,12c,15c fats consist mainly of esters of glycerol (i .e ., triglycerides) with (α-linolenic acid), other isomers of 18:3 may be included . fatty acids of 10 to 22 carbon atoms . The four fatty acids with the • The acid 20:5 6c,9c,12c,15c,17c, which is prevalent in highest concentration are given for each oil; concentrations are many fish oils, is often abbreviated as 20:5 ω-3 or 20:5 given in weight percent . Because there is often a wide variation in n-3 . composition depending on the source of the oil sample, a range (or sometimes an average) is generally given . More complete data on The assistance of Frank D . Gunstone in preparing this table is composition, including minor fatty acids, sterols, and tocopherols, gratefully acknowledged . can be found in the references . The acids are labeled by the codes described in the previous table, “Properties of Fatty Acids and Their Methyl Esters,” which References gives the systematic and common names of the acids . Thus 18:2 1 . Firestone, D ., Physical and Chemical Characteristics of Oils, Fats, and 9c,12c indicates a C18 acid with two double bonds in the 9 and 12 Waxes, 2nd Edition, AOCS Press, Urbana, IL, 2006 .