SUMMARY It Is Shown That Electrical Conductivity Measurement Is a Good Method to Characterize Corrosion Processes That Occur On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interstellar Ices and Radiation-Induced Oxidations of Alcohols
The Astrophysical Journal, 857:89 (8pp), 2018 April 20 https://doi.org/10.3847/1538-4357/aab708 © 2018. The American Astronomical Society. All rights reserved. Interstellar Ices and Radiation-induced Oxidations of Alcohols R. L. Hudson and M. H. Moore Astrochemistry Laboratory, NASA Goddard Space Flight Center, Greenbelt, Maryland, 20771, USA; [email protected] Received 2017 May 2; revised 2018 March 8; accepted 2018 March 9; published 2018 April 18 Abstract Infrared spectra of ices containing alcohols that are known or potential interstellar molecules are examined before and after irradiation with 1 MeV protons at ∼20 K. The low-temperature oxidation (hydrogen loss) of six alcohols is followed, and conclusions are drawn based on the results. The formation of reaction products is discussed in terms of the literature on the radiation chemistry of alcohols and a systematic variation in their structures. The results from these new laboratory measurements are then applied to a recent study of propargyl alcohol. Connections are drawn between known interstellar molecules, and several new reaction products in interstellar ices are predicted. Key words: astrochemistry – infrared: ISM – ISM: molecules – molecular data – molecular processes 1. Introduction 668 cm−1 was produced in solid propargyl alcohol’sIR spectrum after the compound was irradiated with 2 keV Interstellar ices are now recognized as an important comp- electrons (Sivaraman et al. 2015, hereafter SMSB from the onent of the interstellar medium (ISM). The results of multiple coauthors final initials). That IR peak was assigned to benzene infrared (IR) surveys have led to over a dozen assignments of (C H ), which was said “to be the major product from spectral features to molecular ices, with the more abundant 6 6 propargyl alcohol irradiation.” However, a full mid-IR interstellar ices being H O, CO, and CO (Boogert et al. -

Adducts of Propargyl Alcohol and Their Use As Corrosion Inhibitors in Acidizing Systems
Europaisches Patentamt 19) s European Patent Office © Publication number: 0 239 770 Office europeen des brevets A1 © EUROPEAN PATENT APPLICATION © Application number: 87102356.0 © Int. CI.3: C 23 F 11/12 E 21 B 41/02, E 21 B 37/06 © Date of filing: 19.02.87 © Priority: 28.02.86 US 834526 © Applicant: BASF Corporation 9 Campus Drive Parsippany, NJ 07054(US) © Date of publication of application: 07.10.87 Bulletin 87/41 © Inventor: Perry, Christine 22335 Nixon © Designated Contracting States: Riverview Michigan 481 92(US) DE FR GB NL © Inventor: Crema,Stefano Carlo 1874 20th Street Wyandotte Michigan 48192(US) © Inventor: Davis, Pauls 30027 White Gibraltar Michigan 48173(US) @ Representative: Holler, Klaus, Dr. et al, BASF Aktiengesellschaft Carl-Bosch-Strasse 38 D-6700 Ludwigshafen(DE) © Adducts of propargyl alcohol and their use as corrosion inhibitors in acidizing systems. © The invention relates to an acidizing system comprising: (a) an acidizing solution; and (b) an effective corrosion inhibiting amount of a prop- argyl alcohol adduct having the following structural formula I: _ _ HC a C-CH2-0- -(CH2)5C-^ -H (I) wherein n = 1 or 3 and R = H if n = 3 and R = CH3 if n = 1 ; and x = 1 to 5. Corrosion of ferrous metals is inhibited by treating the surface thereof with the acidizing system. o IN M CM 0. Ul Croydon Printing Company Ltd. BASF Corporation ADDUCTS OF PROPARGYL ALCOHOL AND THEIR USE AS CORROSION INHIBITORS IN ACIDIZING SYSTEMS This invention relates to acidizing systems for oil and gas recovery containing propylene oxide and/or butylene oxide adducts of propargyl alcohol as corrosion inhibitors. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Synthesis of Propargyl Alcohol Author(S)
Studies on ethinylation reactions, II : synthesis of propargyl Title alcohol Author(s) Suzuki, Keizo The Review of Physical Chemistry of Japan (1954), 23(2): 66- Citation 72 Issue Date 1954-02-15 URL http://hdl.handle.net/2433/46699 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 23 No. 2 (1953) STUDIES ON ETHINYLATION REACTIONS, II Synthesis of Propargyl Alcohol By Ka¢c~ Sczcxi Introduction Propargyl alcohol is usually produced as the intermediate when butynediol-1, 4 is synthesized from acetylene and fornaldehyde, but it is to be possible to obtain propargyl alcohol as the main product if the conversion to butynediol can be checked by control- ling suitably the reaction conditions. W. Reppeil has obtained the satisfactory results in the synthesis of propargyl alcohol in a continuous process, while the unsuccessful result has been reported so far as a continuous process is adoptedzl. Therefore, it is expected that the synthesis of propargyl alcohol as the main product is difficult in com- parison with the case of butynediol. From the previous studies on the kinetics of the reaction of acetylene with aqueous formaldehyde solutiona~^>and the synthesis of butynediol in a continuous process sl, the results of the investigations on the propargyl alcohol synthesis are summarized as follows. The rate, the apparent equilibrium concentration of propargyl alcohol and the ratio in moles of the quantity of the propargyl alcohol formed to that of formaldehyde consumed, PfF increase when acetylene pressure is raised. In the cases where pH is low and the methanol content contained in an aqueous formaldehyde solution is high, the concentration of propargyl alcohol formed and P/F come to exceed the cases where pH is high and the methanol content is low, though the reaction rate is slow at the earlier stage. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Predicting OH Stretching Fundamental Wavenumbers of Alcohols for Conformational Cite This: Phys
PCCP View Article Online PAPER View Journal | View Issue Predicting OH stretching fundamental wavenumbers of alcohols for conformational Cite this: Phys. Chem. Chem. Phys., 2021, 23, 5629 assignment: different correction patterns for density functional and wave-function-based methods† Robert Medel * and Martin A. Suhm A model is presented for the prediction of OH stretching fundamental wavenumbers of alcohol conformers in the gas phase by application of a small set of empirical anharmonicity corrections to calculations in the harmonic approximation. In contrast to the popular application of a uniform scaling factor, the local chemical structure of the alcohol is taken into account to greatly improve accuracy. Interestingly, different correction patterns emerge for results of hybrid density functional (B3LYP-D3 and PBE0-D3) and wave-function-based Creative Commons Attribution 3.0 Unported Licence. methods (SCS-LMP2, LCCSD(T*)-F12a and CCSD(T)-F12a 1D). This raises questions about electronic structure deficiencies in these methods and differences in anharmonicity between alcohols. After its initial construction on the basis of literature assignments the model is tested with Raman jet spectroscopy of propargyl alcohol, Received 24th January 2021, cyclohexanol, borneol, isopinocampheol and 2-methylbutan-2-ol. For propargyl alcohol a spectral splitting Accepted 24th February 2021 attributed to tunneling is resolved. PBE0-D3 is identified as a well performing and broadly affordable À1 DOI: 10.1039/d1cp00342a electronic structure method for this model. A mean absolute error of 1.3 cm and a maximum absolute error of 3 cmÀ1 result for 46 conformers of 24 alcohols in a 60 cmÀ1 range, when a single parameter is rsc.li/pccp adjusted separately for each alcohol substitution class(methanol,primary,secondary,tertiary). -

Synthesis and Cytotoxicity Evaluation of Small
Synthesis and Cytotoxicity evaluation of small 1,4 - triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations Shiva Kalhor Monfared To cite this version: Shiva Kalhor Monfared. Synthesis and Cytotoxicity evaluation of small 1,4 - triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations. Organic chemistry. Université Pierre et Marie Curie - Paris VI, 2014. English. NNT : 2014PA066235. tel- 01133657 HAL Id: tel-01133657 https://tel.archives-ouvertes.fr/tel-01133657 Submitted on 20 Mar 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE DE DOCTORAT DE L’UNIVERSITE PIERRE ET MARIE CURIE Spécialité Chimie Organique Ecole doctorale de Chimie Moléculaire Paris Centre Présentée par Mme Shiva Kalhor-Monfared Pour obtenir le grade de DOCTEUR de l’UNIVERSITÉ PIERRE ET MARIE CURIE Sujet de la thèse : Synthesis and Cytotoxicity evaluation of small 1,4-triazolic derivatives against B16 melanoma cell lines and a methodolgy study on the synthesis of propargyl ethers from their corresponding propargyl esters without catalyst and under microwave irradiations soutenue le 18 septembre 2014 devant le jury composé de : Mr F. -

Organic Synthesis and Methodology Related to the Malaria Drug Artemisinin Douglas Aaron Engel
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2009 Organic Synthesis and Methodology Related to the Malaria Drug Artemisinin Douglas Aaron Engel Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES ORGANIC SYNTHESIS AND METHODOLOGY RELATED TO THE MALARIA DRUG ARTEMISININ By DOUGLAS A. ENGEL A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Summer Semester, 2009 The members of the committee approve the dissertation of Douglas A. Engel defended on June 30th, 2009. __________________________________ Gregory B. Dudley Professor Directing Dissertation ___________________________________ Thomas Keller Outside Committee Member __________________________________ Marie Krafft Committee Member __________________________________ Lei Zhu Committee Member __________________________________ Geoffery Strouse Committee Member Approved: _____________________________________ Joesph Schlenoff, Chair, Department of Chemistry The Graduate School has verified and approved the above-named committee members. ii This manuscript is dedicated to my family and friends who have supported me through out my life. iii ACKNOWLEDGMENTS Academically, I could not have achieved what I have without the guidance of Dr. Gregory Dudley. It the early years, he was an invaluable source of information and gave me the opportunity to work on some amazing projects. The reactions course he taught my first year in graduate school was probably the most educational course I have ever taken. In the later years, despite my protests, he has forced me to draw my own conclusions and rarely relinquishes an answer to a question I am capable of determining the answer to. -
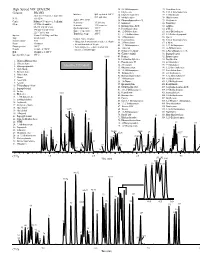
GC and GC/MS Applications Environmental-Volatiles
GC and GC/MS Applications Environmental-Volatiles High Speed VOC EPA 8260 32. 2,2-Dichloropropane 75. Tetrachloroethene Column: DB-VRX 33. Ethyl acetate 76. 1,1,1,2-Tetrachloroethane Interface: Split injector at 150°C, 34. Ethyl-tert-butyl ether 77. 1-Chlorohexane 20 m x 0.18 mm id, 1.0 µm film 60:1 split ratio P/N: 121-1524 35. Methyl acrylate 78. Chlorobenzene Agilent 5973 MSD 36. Dibromofluoromethane IS 79. Ethylbenzene Carrier: Helium at 55 cm/sec (1.5 mL/min) Scan range: 35-260 amu Oven: 45°C for 3.0 minutes 37. Isobutanol 80. Bromoform Scan rate: 3.25 scans/sec 38. Dichloroethane-d4 IS 81. m-Xylene 45-190°C at 36°C/min Quad temperature: 150°C 190-225°C at 20°C/min 39. Pentafluorobenzene 82. p-Xylene Source temperature: 200°C 40. 1,2-Dichloroethane 83. trans-Dichlorobutene 225°C for 0.5 min Transfer line temp: 200°C Injector: Tekmar 3100 Purge and Trap 41. 1,1,1-Trichloroethane 84. 1,3-Dichloro-2-propanol 42. 1-Chlorobutane 85. Styrene Trap: Vocarb 3000 Sample Concentration Sample volume: 5 mL 43. Crotonaldehyde 86. 1,1,2,2-Tetrachloroethane • Halogentated and aromatic analytes at 40 ppb 44. 2-Chloroethanol 87. o-Xylene Purge: 11 minutes • Internal standards at 20 ppb Desorb preheat: 245°C 45. 1,1-Dichloropropene 88. 1,2,3-Trichlropropane • Polar analytes (i.e., ethers, alcohols and 46. 1-Butanol 89. cis-Dichlorobutene Desorb: 1 minute at 250°C ketones at 100-800 ppb) Bake: 10 minutes at 260°C 47. -

Acetylene in Organic Synthesis: Recent Progress and New Uses
Review Acetylene in Organic Synthesis: Recent Progress and New Uses Vladimir V. Voronin 1, Maria S. Ledovskaya 1, Alexander S. Bogachenkov 1, Konstantin S. Rodygin 1 and Valentine P. Ananikov 1,2,* 1 Institute of Chemistry, Saint Petersburg State University, Universitetsky prospect 26, Peterhof 198504, Russia; [email protected] (V.V.V.); [email protected] (M.S.L.); [email protected] (A.S.B.); [email protected] (K.S.R.) 2 N. D. Zelinsky Institute of Organic Chemistry Russian Academy of Sciences, Leninsky prospect 47, Moscow 119991, Russia * Correspondence: [email protected] Received: 16 August 2018; Accepted: 17 September 2018; Published: 24 September 2018 Abstract: Recent progress in the leading synthetic applications of acetylene is discussed from the prospect of rapid development and novel opportunities. A diversity of reactions involving the acetylene molecule to carry out vinylation processes, cross-coupling reactions, synthesis of substituted alkynes, preparation of heterocycles and the construction of a number of functionalized molecules with different levels of molecular complexity were recently studied. Of particular importance is the utilization of acetylene in the synthesis of pharmaceutical substances and drugs. The increasing interest in acetylene and its involvement in organic transformations highlights a fascinating renaissance of this simplest alkyne molecule. Keywords: acetylene; vinylation; cross-coupling; addition reactions; drugs; pharmaceutical substances; biologically active molecule; monomers; polymers 1. Introduction Since the discovery of acetylene, new areas of acetylene chemistry have been continuously developed. The rich scope of chemical transformations available for a C≡C triple bond can be exemplified by coupling [1–5] and addition reactions [6,7]. -

Nomination Background: Propargyl Alcohol (CASRN: 107-19-7)
SUMMARY OF DATA FOR CHEMICAL SELECTION PROPARGYL ALCOHOL CAS No. 107-19-7 BASIS OF NOMINATION TO THE CSWG The nomination of propargyl alcohol to the CSWG is based on high production volume, exposure potential, and suspicion of potential carcinogenicity. Dr. Elizabeth Weisburger, a member of the American Conference of Governmental Industrial Hygienists (ACGIH) TLV Committee as well as the Chemical Selection Working Group (CSWG), provided a list of 281 chemical substances with ACGIH recommended TLVs for which there were no long-term studies cited in the supporting data and no designations with respect to carcinogenicity. She presented the list to the Chemical Selection Planning Group (CSPG) for evaluation as chemicals which may warrant chronic testing; it was affirmed at the CSPG meeting held on August 9, 1994, that the 281 "TLV Chemicals" be reviewed as a Class Study. As a result of the class study review, propargyl alcohol is presented as a candidate for testing by the National Toxicology Program because of: • potential for occupational exposures based on moderately high production volume • evidence of occupational exposures based on TLV and other literature documentation • potential for human exposures based on hazardous waste occurrences in environmental media • suspicion of carcinogenicity based on mixed results in available short-term assays for genetic toxicity and liver and kidney changes in subchronic mammalian toxicity studies • lack of chronic toxicity data. Sources of human exposure to propargyl alcohol are mainly occupational; and the exposure potential is considered moderate based on an estimated U.S. annual production volume range of 0.5 to 2.8 million pounds, an estimate of 54,358 worker exposures (19,933 female) reported in the NOES database, and characterization of propargyl alcohol as a moderately volatile liquid identified in air, water, and soil. -

Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions
inorganics Article Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions Hoang Ngan Nguyen 1, Naoto Tashima 1, Takao Ikariya 1 and Shigeki Kuwata 1,2,* ID 1 Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 2-12-1 E4-1 O-okayama, Meguro-ku, Tokyo 152-8552, Japan; [email protected] (H.N.N.); [email protected] (N.T.); [email protected] (T.I.) 2 Precursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan * Correspondence: [email protected]; Tel.: +81-3-5734-2150 Received: 28 October 2017; Accepted: 14 November 2017; Published: 16 November 2017 Abstract: Propargyl alcohol is a useful synthon in synthetic organic chemistry. We found that the 5 ruthenium(II) complex [Cp*RuCl(diene)] (Cp* = η -C5Me5; diene = isoprene or 1,5-cyclooctadiene (cod)) catalyzes dimerization of 1,1-diphenylprop-2-yn-1-ol (1,1-diphenylpropargyl alcohol, 1a) at room temperature to afford an alkylidenebenzocyclobutenyl alcohol 2a quantitatively. Meanwhile, a stoichiometric reaction of the related hydrido complex [Cp*RuH(cod)] with 1a at 50 ◦C led to isolation of a ruthenocene derivative 4 bearing a cyclopentadienyl ring generated by dehydrogenative trimerization of 1a. Detailed structures of 2a and 4 were determined by X-ray crystallography. The reaction mechanisms for the formation of 2a and 4 were proposed. Keywords: ruthenium; alkyne; propargyl alcohol; C–H cleavage; benzocyclobutene 1. Introduction Propargyl alcohol and their derivatives have been attractive starting materials in synthetic organic chemistry [1–7].