Nucleoid Size Scaling and Intracellular Organization of Translation Across
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Nature of Genomes Viral Genomes Prokaryotic Genome
The nature of genomes • Genomics: study of structure and function of genomes • Genome size – variable, by orders of magnitude – number of genes roughly proportional to genome size • Plasmids – symbiotic DNA molecules, not essential – mostly circular in prokaryotes • Organellar DNA – chloroplast, mitochondrion – derived by endosymbiosis from bacterial ancestors Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Viral genomes • Nonliving particle In prokaryotes, viruses are – nucleic acid sometimes referred to as – protein bacteriophages. • DNA or RNA – single-stranded or double-stranded – linear or circular • Compact genomes with little spacer DNA Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Prokaryotic genome • Usually circular double helix – occupies nucleoid region of cell – attached to plasma membrane • Genes are close together with little intergenic spacer • Operon – tandem cluster of coordinately regulated genes – transcribed as single mRNA • Introns very rare Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company 1 Eukaryotic nuclear genomes • Each species has characteristic chromosome number • Genes are segments of nuclear chromosomes • Ploidy refers to number of complete sets of chromosomes –haploid (1n): one complete set of genes – diploid (2n) – polyploid (≥3n) • In diploids, chromosomes come in homologous pairs (homologs) In humans, somatic cells have – structurally similar 2n = 46 chromosomes. – same sequence of genes – may contain different alleles Chapter 2: Genes and genomes © 2002 by W. H. -

The Splicing Factor XAB2 Interacts with ERCC1-XPF and XPG for RNA-Loop Processing During Mammalian Development
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.20.211441; this version posted July 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The Splicing Factor XAB2 interacts with ERCC1-XPF and XPG for RNA-loop processing during mammalian development Evi Goulielmaki1*, Maria Tsekrekou1,2*, Nikos Batsiotos1,2, Mariana Ascensão-Ferreira3, Eleftheria Ledaki1, Kalliopi Stratigi1, Georgia Chatzinikolaou1, Pantelis Topalis1, Theodore Kosteas1, Janine Altmüller4, Jeroen A. Demmers5, Nuno L. Barbosa-Morais3, George A. Garinis1,2* 1. Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology- Hellas, GR70013, Heraklion, Crete, Greece, 2. Department of Biology, University of Crete, Heraklion, Crete, Greece, 3. Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina da Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028 Lisboa, Portugal, 4. Cologne Center for Genomics (CCG), Institute for Genetics, University of Cologne, 50931, Cologne, Germany, 5. Proteomics Center, Netherlands Proteomics Center, and Department of Biochemistry, Erasmus University Medical Center, the Netherlands. Corresponding author: George A. Garinis ([email protected]) *: equally contributing authors bioRxiv preprint doi: https://doi.org/10.1101/2020.07.20.211441; this version posted July 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract RNA splicing, transcription and the DNA damage response are intriguingly linked in mammals but the underlying mechanisms remain poorly understood. Using an in vivo biotinylation tagging approach in mice, we show that the splicing factor XAB2 interacts with the core spliceosome and that it binds to spliceosomal U4 and U6 snRNAs and pre-mRNAs in developing livers. -
Chapter 4 – Cell Structure
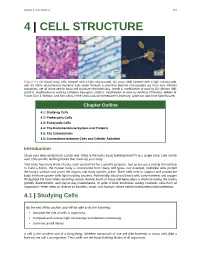
Chapter 4 | Cell Structure 107 4 | CELL STRUCTURE Figure 4.1 (a) Nasal sinus cells (viewed with a light microscope), (b) onion cells (viewed with a light microscope), and (c) Vibrio tasmaniensis bacterial cells (seen through a scanning electron microscope) are from very different organisms, yet all share certain basic cell structure characteristics. (credit a: modification of work by Ed Uthman, MD; credit b: modification of work by Umberto Salvagnin; credit c: modification of work by Anthony D'Onofrio, William H. Fowle, Eric J. Stewart, and Kim Lewis of the Lewis Lab at Northeastern University; scale-bar data from Matt Russell) Chapter Outline 4.1: Studying Cells 4.2: Prokaryotic Cells 4.3: Eukaryotic Cells 4.4: The Endomembrane System and Proteins 4.5: The Cytoskeleton 4.6: Connections between Cells and Cellular Activities Introduction Close your eyes and picture a brick wall. What is the wall's basic building block? It is a single brick. Like a brick wall, cells are the building blocks that make up your body. Your body has many kinds of cells, each specialized for a specific purpose. Just as we use a variety of materials to build a home, the human body is constructed from many cell types. For example, epithelial cells protect the body's surface and cover the organs and body cavities within. Bone cells help to support and protect the body. Immune system cells fight invading bacteria. Additionally, blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide. Each of these cell types plays a vital role during the body's growth, development, and day-to-day maintenance. -

The Mitochondrial Genome. the Nucleoid
ISSN 0006-2979, Biochemistry (Moscow), 2016, Vol. 81, No. 10, pp. 1057-1065. © Pleiades Publishing, Ltd., 2016. Original Russian Text © A. A. Kolesnikov, 2016, published in Biokhimiya, 2016, Vol. 81, No. 10, pp. 1322-1331. REVIEW The Mitochondrial Genome. The Nucleoid A. A. Kolesnikov Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: [email protected] Received May 30, 2016 Revision received July 1, 2016 Abstract—Mitochondrial DNA (mtDNA) in cells is organized in nucleoids containing DNA and various proteins. This review discusses questions of organization and structural dynamics of nucleoids as well as their protein components. The structures of mt-nucleoid from different organisms are compared. The currently accepted model of nucleoid organization is described and questions needing answers for better understanding of the fine mechanisms of the mitochondrial genetic apparatus functioning are discussed. DOI: 10.1134/S0006297916100047 Key words: mitochondrial nucleoid, mitochondrial genome It is now clear that understanding of genome organ- mtDNA). In fact, proteins associated with a nucleoid can ization of subcellular structures is necessary for under- influence the speed of accumulation of mutations. standing of cellular processes occurring through the cell In the last decade, reviews devoted to this subject cycle, including questions of storage, realization, and appeared regularly [5-9]. Generally, they cover the subject transmission of genetic information. A considerable of nucleoid organization in metazoan mitochondria amount of information about forms and sizes of mito- (mainly in human cells) and fungi (mainly yeast). chondrial DNA (mtDNA) throughout evolution has been Reviews touching plant cells are much less frequent [10, accumulated [1]. -

Nucleolus: a Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky
Nucleolus: A Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky To cite this version: Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, et al.. Nucleolus: A Central Hub for Nuclear Functions. Trends in Cell Biology, Elsevier, 2019, 29 (8), pp.647-659. 10.1016/j.tcb.2019.04.003. hal-02322927 HAL Id: hal-02322927 https://hal.archives-ouvertes.fr/hal-02322927 Submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Nucleolus: A Central Hub for Nuclear Functions Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, Sergey Razin, Yegor Vassetzky To cite this version: Olga Iarovaia, Elizaveta Minina, Eugene Sheval, Daria Onichtchouk, Svetlana Dokudovskaya, et al.. Nucleolus: A Central Hub for Nuclear Functions. Trends in Cell Biology, Elsevier, 2019, 29 (8), pp.647-659. 10.1016/j.tcb.2019.04.003. hal-02322927 HAL Id: hal-02322927 https://hal.archives-ouvertes.fr/hal-02322927 Submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. -

Unit 6-Nucleus
Unit 6-Nucleus Dr. Pallee shree Nucleus • Nucleus is the most important organelle in the cell • It distinguishes eukaryotic from prokaryotic cells • By housing the cell's genome, the nucleus serves both as the repository of genetic information and as the cell's control center • DNA replication, transcription, and RNA processing all take place within the nucleus Cont… • A nucleus is a double-membraned eukaryotic cell organelle that contains the genetic material. • It appears in an oval shape averages 5µm in width. • It often lies in the centre of a cell • The nucleus was the first organelle to be discovered • Nuclei 1st discovered and named by Robert Brown • Role of nucleus 1st demonestrated by Max Hammerling Ultra structure of Nucleus 1. Nuclear envelope 2. nuclear pores 3. Nucleoplasm 4. Nucleolus 5. Chromosomes 1. Structure of Nuclear envelope • The nuclear envelope has a complex structure consisting of a) Two nuclear membranes separated by a perinuclear space measuring about 20–40 nm across b) Underlying nuclear lamina • The nucleus is surrounded by a system of two concentric membranes, called the inner and outer nuclear membranes • The inner and outer nuclear membranes are joined at nuclear pore complexes a. Nuclear membranes • The outer nuclear membrane is continuous with the endoplasmic reticulum, so the space between the • The critical function of the inner and outer nuclear membranes nuclear membranes is to act as is directly connected with the lumen a barrier that separates the of the ER contents of the nucleus from the cytoplasm. • It is functionally similar to the membranes of the ER and has • Like other cell membranes, ribosomes bound to its cytoplasmic each nuclear membrane is a surface but protein composition phospholipid bilayer differ slightly as they are enriched in permeable only to small proteins which binds to cytoskeleton nonpolar molecules • The inner nuclear membrane carries • Other molecules are unable to proteins that are specific to the diffuse through the bilayer. -

Functional Proximity Mapping of RNA Binding Proteins Uncovers a Mitochondrial Mrna Anchor That Promotes Stress Recovery
Functional proximity mapping of RNA binding proteins uncovers a mitochondrial mRNA anchor that promotes stress recovery Wei Qin Stanford University Samuel Myers Broad Institute Dominique Carey The Broad Institute of MIT and Harvard Steven Carr Broad Institute Alice Ting ( [email protected] ) Stanford University https://orcid.org/0000-0002-8277-5226 Article Keywords: Unbiased Proteome Discovery, Nucleolus, Outer Mitochondrial Membrane, Local Translation, Functional Protein Mapping Posted Date: November 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-103196/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/49 Abstract Proximity labeling (PL) with genetically-targeted promiscuous enzymes has emerged as a powerful tool for unbiased proteome discovery. By combining the spatiotemporal specicity of PL with methods for functional protein enrichment, it should be possible to map specic protein subclasses within distinct compartments of living cells. Here we demonstrate this capability for RNA binding proteins (RBPs), by combining peroxidase-based PL with organic-aqueous phase separation of crosslinked protein-RNA complexes (“APEX-PS”). We validated APEX-PS by mapping nuclear RBPs, then applied it to uncover the RBPomes of two unpuriable subcompartments - the nucleolus and the outer mitochondrial membrane (OMM). At the OMM, we discovered the RBP SYNJ2BP, which retains specic nuclear-encoded mitochondrial mRNAs during translation stress, to promote their local translation and import of protein products into the mitochondrion during stress recovery. APEX-PS is a versatile tool for compartment- specic RBP discovery and expands the scope of PL to functional protein mapping. Introduction RNA-protein interactions are pervasive in both transient and stable macromolecular complexes underlying transcription, translation and stress response 1, 2. -
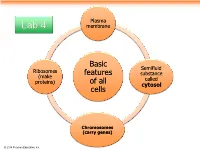
Basic Features of All Cells
Plasma Lab 4 membrane Basic Semifluid Ribosomes features substance (make called proteins) of all cytosol cells Chromosomes (carry genes) © 2014 Pearson Education, Inc. Prokaryotic cells are characterized by having . No nucleus . DNA in an unbound region called the nucleoid . No membrane-bound organelles . Cytoplasm bound by the plasma membrane © 2014 Pearson Education, Inc. Eukaryotic cells are characterized by having • DNA in a nucleus that is bounded by a membranous nuclear envelope • Membrane-bound organelles • Cytoplasm in the region between the plasma membrane and nucleus Eukaryotic cells are generally much larger than prokaryotic cells © 2014 Pearson Education, Inc. Figure 6.8a ENDOPLASMIC RETICULUM (ER) Nuclear envelope Rough ER Smooth ER Nucleolus NUCLEUS Flagellum Chromatin Centrosome Plasma membrane CYTOSKELETON: Microfilaments Intermediate filaments Microtubules Ribosomes Microvilli Golgi apparatus Peroxisome Lysosome Mitochondrion © 2014 Pearson Education, Inc. Figure 6.8b Nuclear envelope NUCLEUS Nucleolus Rough ER Chromatin Smooth ER Ribosomes Golgi Central vacuole apparatus Microfilaments CYTOSKELETON Microtubules Mitochondrion Peroxisome Plasma Chloroplast membrane Cell wall Plasmodesmata Wall of adjacent cell © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. Table 6.1 © 2014 Pearson Education, Inc. Cell Walls of Plants The cell wall is an extracellular structure that distinguishes plant cells from animal cells Plant cell walls Prokaryotes, are made -

Coli Chromosome Into a Nucleoid Filament
Strong intranucleoid interactions organize the Escherichia coli chromosome into a nucleoid filament Paul A. Wigginsa,1, Keith C. Cheverallsa,b, Joshua S. Martina,b, Robert Lintnera, and Jané Kondevb aWhitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142; and bMartin A. Fisher School of Physics, Brandeis University, 415 South Street, Waltham, MA 02453 Edited by Nancy E Kleckner, Harvard University, Cambridge, MA, and approved January 27, 2010 (received for review October 26, 2009) The stochasticity of chromosome organization was investigated by Our quantitative measurements of E. coli nucleoid structure con- fluorescently labeling genetic loci in live Escherichia coli cells. In firm this qualitative picture: The body of the nucleoid is linearly spite of the common assumption that the chromosome is well organized along the long-axis of the cell, implying that the modeled by an unstructured polymer, measurements of the locus nucleoid has a nearly-constant linear packing density, except distributions reveal that the E. coli chromosome is precisely orga- for a short region, genomically ter-proximate, which connects nized into a nucleoid filament with a linear order. Loci in the body the two arms of the chromosome (11). But in spite of the obser- of the nucleoid show a precision of positioning within the cell of vation of a linear chromosome organization in both E. coli and better than 10% of the cell length. The precision of interlocus dis- C. crescentus, the mechanism which gives rise to this characteristic tance of genomically-proximate loci was better than 4% of the cell structure is unknown. length. The measured dependence of the precision of interlocus To probe the mechanism of nucleoid organization, we measure distance on genomic distance singles out intranucleoid interactions and analyze the position fluctuations of single loci and the cor- as the mechanism responsible for chromosome organization. -

Driving Nucleolar Assembly
Downloaded from genesdev.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE Driving nucleolar assembly Kathleen L. McCann,1 and Susan J. Baserga1,2,3,4 1Department of Genetics, 2Department of Molecular Biophysics and Biochemistry, 3Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, Connecticut 06520, USA In this issue of Genes & Development,Grobandcol- breaks down (open mitosis), transcription of the ribosomal leagues (pp. 220–230) identify the minimal molecular re- RNA (rRNA) is inhibited, and the nucleolus disassem- quirements to assemble a fully functional nucleolus in bles. Upon completion of mitosis, rRNA transcription human cells and demonstrate the importance of the is reinitiated within the NOR, ribosome biogenesis fac- nucleolar transcription factor upstream binding factor tors are recruited, and the nucleolus is assembled. Only (UBF) as a mitotic bookmark at the ribosomal DNA NORs that are actively engaged in transcription can (rDNA). direct nucleolar assembly (Hernandez-Verdun 2011). Consequently, the nucleolus truly appears to be ‘‘an organelle formed by the act of building a ribosome’’ (Melese and Xue 1995). In all eukaryotes, the nucleolus is an essential, non- To begin to reveal the molecular mechanisms of nucle- membrane-bound organelle within the nucleus. Assem- olar formation, McStay’s laboratory (Mais et al. 2005; Grob bled around the ribosomal DNA (rDNA), the nucleolus et al. 2014) has applied synthetic biology. In an earlier is the site of ribosome biogenesis. In addition to its role report, they introduced 6.4 kb of DNA repeat sequences in making ribosomes, the nucleolus functions in other from the intergenic spacer of the Xenopus ribosomal gene important cellular processes, including stress sensing, into a noncanonical site in the human genome. -

The Chloroplast Nucleoid in Ochromonas Danica I
J. Cell Sci. 16, 557-577 0974) 557 Printed in Great Britain THE CHLOROPLAST NUCLEOID IN OCHROMONAS DANICA I. THREE-DIMENSIONAL MORPHOLOGY IN LIGHT- AND DARK-GROWN CELLS SARAH P. GIBBS, D. CHENG AND T. SLANKIS Department of Biology, McGill University, Montreal HT,C 3G1, Canada SUMMARY The 3-dimensional structure of the plastid nucleoid was determined from serial sections of the plastid of dark-grown, greening, and light-grown cells of Ochromonas danica. In light- grown and greening cells, the chloroplast nucleoid forms a continuous cord or ring which closely follows the rim of each lateral lobe of the chloroplast and is continuous across the top and bottom of the bridge connecting the 2 chloroplast lobes. The nucleoid always lies just inside the chloroplast girdle bands where they loop around the rim of the plastid. It was demonstrated by electron-microscopic autoradiography of greening cells labelled with [3H]- thymidine that all the plastid DNA is localized in this peripheral ring-shaped nucleoid. In the proplastid of dark-grown cells, the nucleoid also forms a ring-shaped structure lying just inside the single girdle thylakoid, although frequent irregularities, such as gaps, are present. It is postulated that the girdle bands determine the shape of the chloroplast nucleoid, possibly by having specific attachment sites for the plastid DNA molecules. A survey of the literature shows that a peripheral ring-shaped chloroplast nucleoid is a characteristic feature of the 5 classes of algae whose chloroplasts possess girdle bands, namely the Raphidophyceae, Chryso- phyceae, Bacillariophyceae, Xanthophyceae, and Phaeophyceae, and has never been observed in plants whose plastids lack girdle bands. -

Segregation of the Nucleolus Produced by Anthramycin1
[CANCER RESEARCH 28, 81-90, January 1968] Segregation of the Nucleolus Produced by Anthramycin1 Curtis Harris, Harold Grady, and Donald Svoboda Department oj Pathology, University oj Kansas Medical Center, Kansas City, Kansas 66103 SUMMARY mycin does not produce toxicity in the bone marrow and gastrointestinal tract (49). It possesses certain structural fea Anthramycin, an antibiotic possessing antitumor properties, tures in common with actinomycin D and the postulated biosyn- was administered to rats and mice in a single intraperitoneal thetic intermediates of the actinomycin^, 3-hydroxy-4-methyI- close ranging from 1 to 10 mg/kg body weight. Light micro anthraniloyl peptides (3,47). Like kanamycin, neomycin, and scopy showed that, at the highest dose, anthramycin produced other antibiotics (48), anthramycin permanently bleaches necrosis in the pancreas of rats but not in mice. By electron Euglena gracilis (11). microscopy, nucleolar changes were observed in the spleen and This report demonstrates that anthramycin is another agent kidney of both species by 1 hour. Acinar cells of the pancreas which causes nucleolar changes in pancreatic cells, reticulo showed nucleolar caps and segregation of nucleolar constituents endothelial cells of liver and spleen, and in proximal tubular in both species at 3 to 4 hours. By 10 hours segregation of the epithelium in the kidney. nucleolus and focal cytoplasmic degradation were prominent in pancreatic cells of rats given higher doses but were not MATERIALS AND METHODS observed in hepatocytes of either species. Anthramycin produced nucleolar alterations in Kupffer cells, Thirty-six inbred Fisher-344 male rats weighing between 95 endocrine and exocrine pancreatic cells, proximal tubular cells and 240 gm and fifteen C57 black mice weighing between 20 of the kidney, and in the reticuloendothelial cells of the spleen and 30 gm were used.