Takotsubo Cardiomyopathy in Dextrocardia with Situs Inversus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

EKG Workshop Texas Academy of Physician Assistants 2018 TAPA 43Rd Annual CME Conference
EKG Workshop Texas Academy of Physician Assistants 2018 TAPA 43rd Annual CME Conference David J. Klocko, MPAS, PA-C Associate Professor and Academic Coordinator UT Southwestern Department of PA Studies Disclosures Content in this presentation is licensed under a Creative Commons Attribution 4.0 International license. David J. Klocko is receiving a stipend from TAPA to do this workshop. Objectives Review basic electrophysiology. Recognize common cardiac dysrhythmias. Recognize common conductive disorders. Review axis, bundle branch block, chamber enlargement and hypertrophy. Recognize common ECG changes associated with myocardial ischemia and infarction. Systematic Evaluation of the 12- lead EKG –Our ultimate goal ! Step Check for: LOOK AT: 1. Rhythm Rhythm Strip 2. Axis I, AvF ** 3. BBB V1, V6 4. Ischemia, Injury or I-AvL, V5-V6 Infarct V1-V4 II, III, AvF 5. Chamber II, V1 Enlargement Intervals & Segments PR interval (PRI): start of atrial depolarization to start of ventricular depolarization Normal: 0.12 – 0.20 sec QRS complex: depolarization of ventricles Normal: 0.04 – 0.11 sec When QRS >0.12 sec, think about an interventricular conduction delay Intervals & Segments ST segment: end ventricular depolarization to start of repolarization QT interval (QTI): both ventricular depolarization & repolarization Normal: 0.3 – 0.4 sec; varies with heartrate Faster heartrates….shorter QTIs; slower heartrates ….longer QTIs Heart Rate For regular rhythms, measure the interval between complexes in large boxes 300-150-100-75-60-50-43-37-33 -

Changes in Cardiac Geometry Due to Hypertrophy
Changes in Cardiac Geometry Due to Hypertrophy By Marta Ellen Pedersen A Master’s Paper Submitted in Partial Fulfillment of The Requirements for the Degree of Master of Science in Clinical Exercise Physiology Dr. Joseph O’ Kroy Date University of Wisconsin-River Falls 2014 Introduction For any given body size, men have larger hearts than women, athletes have larger hearts than nonathletes, and often times, an enlarged heart is a symptom of an underlying disorder that is causing the heart to work harder than normal. This review will emphasize the differences between a pathologically enlarged heart and an athletically enlarged heart. Pathologically induced hypertrophy (myopathy) When heart cells get bigger, (often is the case when heart disease is present) the total heart works less efficiently. Some people suffer from conditions like hypertrophic cardiomyopathy, which includes significant heart muscle enlargement, and can be genetic or caused by high blood pressure. Cardiomyopathy decreases the size of the heart's chambers, reducing blood flow. Hypertrophy, or thickening, of the heart muscle can occur in response to increased stress on the heart. The most common causes of Cardiomyopathy are related to increased blood pressure. The extra work of pumping blood against the increased pressure causes the ventricle to thicken over time, the same way a body muscle increases in mass in response to weightlifting. Cardiomyopathy can occur in both the right and left atrium and the right and left ventricles. Blood travels through the right ventricle to the lungs. If conditions occur that decrease pulmonary circulation, extra stress can be placed on the right ventricle, and can lead to right ventricular myopathy. -

Abnormal ECG Findings in Athletes Normal ECG
SEATTLE CRITERIA Abnormal ECG findings in athletes These ECG findings are unrelated to regular training or expected physiologic adaptation to exercise, may suggest the presence of pathologic cardiovascular disease, and require further diagnostic evaluation. Abnormal ECG finding Definition T wave inversion > 1 mm in depth in two or more leads V2-V6, II and aVF, or I and aVL (excludes III, aVR, and V1) ST segment depression ≥ 0.5 mm in depth in two or more leads Pathologic Q waves > 3 mm in depth or > 40 ms in duration in two or more leads (except III and aVR) Complete left bundle branch block QRS ≥ 120 ms, predominantly negative QRS complex in lead V1 (QS or rS), and upright monophasic R wave in leads I and V6 Intra-ventricular conduction delay Any QRS duration ≥ 140 ms Left axis deviation -30° to -90° Left atrial enlargement Prolonged P wave duration of > 120 ms in leads I or II with negative portion of the P wave ≥ 1 mm in depth and ≥ 40 ms in duration in lead V1 Right ventricular hypertrophy R-V1 + S-V5 > 10.5 mm and right axis deviation > 120° pattern Ventricular pre-excitation PR interval < 120 ms with a delta wave (slurred upstroke in the QRS complex) and wide QRS (> 120 ms) Long QT interval* QTc ≥ 470 ms (male) QTc ≥ 480 ms (female) QTc ≥ 500 ms (marked QT prolongation) Short QT interval* QTc ≤ 320 ms Brugada-like ECG pattern High take-off and downsloping ST segment elevation followed by a negative T wave in ≥ 2 leads in V1-V3 Profound sinus bradycardia < 30 BPM or sinus pauses ≥ 3 sec Mobitz type II 2° AV block Intermittently non-conducted P waves not preceded by PR prolongation and not followed by PR shortening 3° AV block Complete heart block Atrial tachyarrhythmias Supraventricular tachycardia, atrial fibrillation, atrial flutter Premature ventricular contractions ≥ 2 PVCs per 10 second tracing Ventricular arrhythmias Couplets, triplets, and non-sustained ventricular tachycardia *The QT interval corrected for heart rate is ideally measured with heart rates of 60-90 bpm. -

Dysrhythmias
CARDIOVASCULAR DISORDERS DYSRHYTHMIAS I. BASIC PRINCIPLES OF CARDIAC CONDUCTION DISTURBANCES A. Standard ECG and rhythm strips 1. Recordings are obtained at a paper speed of 25 mm/sec. 2. The vertical axis measures distance; the smallest divisions are 1 mm ×1 mm. 3. The horizontal axis measures time; each small division is 0.04 sec/mm. B. Normal morphology Courtesy of Dr. Michael McCrea 1. P wave = atrial depolarization a. Upright in leads I, II, III, aVL, and aVF; inverted in lead aVR b. Measures <0.10 seconds wide and <3 mm high c. Normal PR interval is 0.12–0.20 seconds. 2. QRS complex = ventricular depolarization a. Measures 0.06-0.10 seconds wide b. Q wave (1) <0.04 seconds wide and <3 mm deep (2) Abnormal if it is >3 mm deep or >1/3 of the QRS complex. c. R wave ≤7.5 mm high 3. QT interval varies with rate and sex but is usually 0.33–0.42 seconds; at normal heart rates, it is normally <1/2 the preceding RR interval. 4. T wave = ventricular repolarization a. Upright in leads I, II, V3–V6; inverted in aVR b. Slightly rounded and asymmetric in configuration c. Measures ≤5 mm high in limb leads and ≤10 mm high in the chest leads 5. U wave = a ventricular afterpotential a. Any deflection after the T wave (usually low voltage) b. Same polarity as the T wave c. Most easily detected in lead V3 d. Can be a normal component of the ECG e. Prominent U waves may indicate one of the following: (1) Hypokalemia (<3 mEq/L) (2) Hypercalcemia (3) Therapy with digitalis, phenothiazines, quinidine, epinephrine, inotropic agents, or amiodarone (4) Thyrotoxicosis f. -

Practical Cardiology Review
Practical Cardiology Review Practical Cardiology Review: A Self-assessment Tool Edited by Majid Maleki, Azin Alizadehasl and Majid Haghjoo Practical Cardiology Review: A Self-assessment Tool Edited by Majid Maleki, Azin Alizadehasl and Majid Haghjoo This book first published 2019 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2019 by Majid Maleki, Azin Alizadehasl, Majid Haghjoo and contributors All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-3729-3 ISBN (13): 978-1-5275-3729-3 TABLE OF CONTENTS Preface ....................................................................................................... ix Chapter 1 .................................................................................................... 1 Evaluation of Patients with Cardiovascular Problems Majid Maleki, MD Chapter 2 .................................................................................................... 6 Electrocardiography Mohammad Javad Alemzadeh-Ansari, MD and Mohammadali Sadrameli, MD Chapter 3 .................................................................................................. 66 Exercise Stress Testing Sedigheh Saedi, MD Chapter -

Morphological Abnormalities in Baseline Ecgs in Healthy Normal Volunteers Participating in Phase I Studies
Indian J Med Res 135, March 2012, pp 322-330 Morphological abnormalities in baseline ECGs in healthy normal volunteers participating in phase I studies Pooja Hingorani, Mili Natekar, Sheetal Deshmukh, Dilip R. Karnad, Snehal Kothari, Dhiraj Narula & Yash Lokhandwala Research Section, Quintiles Cardiac Safety Services, Mumbai, India Received September 30, 2010 Background & objectives: Morphological abnormalities in 12-lead electrocardiograms (ECGs) are seen in subgroups of healthy individuals like athletes and air-force personnel. As these populations may not truly represent healthy individuals, we assessed morphological abnormalities in ECG in healthy volunteers participating in phase I studies, who are screened to exclude associated conditions. Methods: ECGs from 62 phase I studies analyzed in a central ECG laboratory were pooled. A single drug-free baseline ECG from each subject was reviewed by experienced cardiologists. ECG intervals were measured on five consecutive beats and morphological abnormalities identified using standard guidelines. Results: Morphological abnormalities were detected in 25.5 per cent of 3978 healthy volunteers (2495 males, 1483 females; aged 18-76 yr); the presence was higher in males (29.3% vs. 19.2% in females; P<0.001). Rhythm abnormalities were the commonest (11.5%) followed by conduction abnormalities (5.9%), axis deviation (4%), ST-T wave changes (3.1%) and chamber enlargement (1.4%). Incomplete right bundle branch block (RBBB), short PR interval and right ventricular hypertrophy were common in young subjects (<20 yr) while atrial fibrillation, first degree atrioventricular block, complete RBBB and left anterior fascicular block were more prevalent in elderly subjects (>65 yr). Prolonged PR interval, RBBB and intraventricular conduction defects were more common in males while sinus tachycardia, short PR interval and non-specific T wave changes were more frequent in females. -
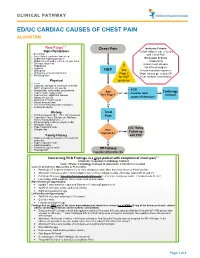
Ed/Uc Cardiac Causes of Chest Pain Algorithm
CLINICAL PATHWAY ED/UC CARDIAC CAUSES OF CHEST PAIN ALGORITHM 1,2 Red Flags Chest Pain Inclusion Criteria Signs/Symptoms Verbal children, age 2-22 yrs • Exertional with Chest Pain • Acute Onset, awakens from sleep • Substernal crushing pressure Exclusion Criteria • Radiation to shoulder, arm, neck, jaw, back Ill appearing • Syncope, dizziness Known heart disease • Palpitations • Dyspnea ! Hx of heart surgery • Orthopnea H&P See Known ingestion/exposure • Pulmonary embolus risk factors Page 2 Major trauma preceding CP • Illicit Drug Use for H&P Acute asthma exacerbation Physical guidance • Fever • Cyanosis, tachypnea, shortness of breath, WOB, abnormal breath sounds • • ECG Bradycardia, tachycardia, dysrhythmia Any Cardiology • Hypertension, hypotension Yes • Consider other • New murmur, significant murmur Red Flags? Consult • Gallop, friction rub causes of chest pain • Abnormal 2nd heart sound • Distant heart sounds • Decreased femoral/peripheral pulses No • Peripheral edema History Treat • Arthritis/vasculitis (SLE, IBD, JIA, Kawasaki) • Connective Tissue Disease (ie. Marfans, Pain Ehlers-Danlos Syndrome…) • QT-prolonging meds (ex. psych meds) • Oncologic history • Hypercoagulable state D/C Home • Dyslipidemia Pain Yes Follow up Improved? Family History with PCP • Sudden unexplained death or MI under 40 years old • Hypercoagulable state No • Cardiomyopathy • Pulmonary hypertension Off Pathway • Prolonged QT Consider Alternative Dx Concerning ECG Findings in a >2yo patient with complaint of chest pain1,2 Anschutz: In-person Cardiology -

Left Bundle Branch Block and Right Axis Deviation Due to Severe Coronary Heart Disease
LEFT BUNDLE BRANCH BLOCK AND RIGHT AXIS DEVIATION DUE TO SEVERE CORONARY HEART DISEASE B L Chia ABSTRACT The simultaneous occurrence of left bundle branch block and right axis deviation is very rare and occurs mainly in patients with cardiomyopathy. This paper describes a 61 -year-old man with end stage ischaemic heart disease who presented with this unique electrocardiographic abnormality. Keywords : Bundle branch block, Coronary disease SINGAPORE MED J 1992; Vol 33: 413-414 CASE REPORT Fig 1 - ECG shows complete left bundle branch block A 61 -year -old Chinese man was admitted to the coronary care as evidenced by a widened ORS complex (0.16 sec) unit for severe, intractable angina occurring at rest. Seven years with a RSr pattern in lead V6. There is coexisting right previously, he had suffered an uncomplicated acute myocar- axis (approximately +140°). The PR interval is prolonged dial infarction. However, the records of this hospitalization are (0.24 sec) and this is most likely due to the digoxin which unavailable. Clinical examination on admission revealed an the patient was taking. apical holosystolic murmur and signs of congestive cardiac failure. The 12 -lead ECG showed evidence of an old anterior Q wave myocardial infarction but no bundle branch block. Serial cardiac enzymes which were done were within the nor- mal limits. Despite repeated intravenous injections of mor- 1 11 AVR AVL AVF phine and oral isosorbide dinitrate, the patient still complained III of severe continuous chest pain. Coronary angiography which was then performed showed: (1) 75% stenosis of the left main coronary artery (2) total occlusion of the left anterior descend- ing artery, circumflex artery and the mid right coronary artery V5 V6 (3) 60% stenosis of the diagonal artery. -
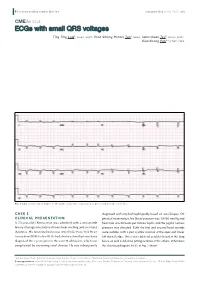
Ecgs with Small QRS Voltages
E lectrocardiography S eries Singapore Med J 2012; 53(5) : 299 CMEARTICLE ECGs with small QRS voltages Ting Ting Low1, MBBS, MRCP, Voon Shiong Ronnie Tan1, MBBS, Swee-Guan Teo1, MBBS, MRCP, Kian-Keong Poh1,2, FRCP, FACC Fig. 1 ECG shows low voltages in the QRS complexes, especially in praecordial leads V1 to V6. CASE 1 diagnosed with amyloid nephropathy based on renal biopsy. On CLINICAL PRESENTATION physical examination, his blood pressure was 105/60 mmHg and A 70-year-old Chinese man was admitted with a one-month heart rate was 80 beats per minute (bpm), and the jugular venous history of progressive bilateral lower limb swelling and exertional pressure was elevated. Both the first and second heart sounds dyspnoea. His functional status was noted to be New York Heart were audible, with a pan-systolic murmur at the apex and lower Association (NYHA) class III. He had a history of multiple myeloma left sternal edge. There were bilateral crackles heard at the lung diagnosed three years prior to this current admission, which was bases, as well as bilateral pitting oedema at the ankles. What does complicated by worsening renal disease. He was subsequently the electrocardiogram (ECG) in Fig. 1 show? 1Cardiac Department, National University Heart Centre, 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore Correspondence: A/Prof Poh Kian Keong, Senior Consultant and Associate Professor, Cardiac Department, National University Heart Centre, 1E Kent Ridge Road, NUHS Tower Block, Level 9, Singapore 119228. [email protected] E lectrocardiography S eries ECG INTERPRETATION The mitral in-flow Doppler signals showed a restrictive pattern. -

Guidelines for the Interpretation of the Neonatal Electrocardiogram
European Heart Journal (2002) 23, 1329–1344 doi:10.1053/euhj.2002.3274, available online at http://www.idealibrary.com on Task Force Report Guidelines for the interpretation of the neonatal electrocardiogram A Task Force of the European Society of Cardiology P. J. Schwartz1 (Chair), A. Garson, Jr2, T. Paul3, M. Stramba-Badiale4, V. L. Vetter5, E. Villain6 and C. Wren7 1Department of Cardiology, University of Pavia and IRCCS Policlinico S. Matteo, Pavia, Italy; 2University of Virginia, Charlottesville, VA, U.S.A.; 3The Children’s Heart Program of South Carolina, Medical University of South Carolina, Charleston, SC, U.S.A.; 4Pediatric Arrhythmias Center, IRCCS Istituto Auxologico Italiano, Milan, Italy; 5Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA, U.S.A.; 6Division of Pediatric Cardiology, Department of Pediatrics, Hoˆpital Necker Enfants Malades, Paris, France; 7Department of Paediatric Cardiology, Freeman Hospital, Newcastle upon Tyne, U.K. Introduction.............................................................1329 Left ventricular hypertrophy............................1338 Normal electrocardiogram in the newborn .............1330 Low QRS voltage.............................................1338 Normal values......................................................1330 Ventricular repolarization....................................1338 Technology ..........................................................1330 QT prolongation: -

EKG Interpretations
EKG Interpretations Chad Link, DO FACC Cardiologist Chairman Cardiology Section Sparrow TCI Lansing, MI Disclosures Objectives • Review general method for EKG interpretation • Review specific points of “data gathering” and “diagnoses” on EKG • Review treatment considerations • Review clinical cases/EKG’s Cardiology/EKG Board Review- MJ Bradley, DO EKG EKG – 12 Leads • Anterior Leads - V1, V2, V3, V4 • Inferior Leads – II, III, aVF • Left Lateral Leads – I, aVL, V5, V6 • Right Leads – aVR, V1 11 Step Method for Reading EKG’s • “Data Gathering” – steps 1-4 – 1. Standardization – make sure paper and paper speed is standardized – 2. Heart Rate – 3. Intervals – PR, QT, QRS width – 4. Axis – normal vs. deviation Cardiology/EKG Board Review- MJ Bradley, DO 11 Step Method for Reading EKG’s • “Diagnoses” – 5. Rhythm – 6. Atrioventricular (AV) Block – 7. Bundle Branch Block or Hemiblock – 8. Preexcitation – 9. Enlargement and Hypertrophy – 10. Coronary Artery Disease – 11. Utter Confusion » The Only EKG Book You’ll Ever Need Malcolm S. Thaler, MD Cardiology/EKG Board Review- MJ Bradley, DO Heart Rate • Regular Rhythms Heart Rate • Irregular Rhythms Intervals • Measure length of PR interval, QT interval, width of P wave, QRS complex QTc • QTc = QT interval corrected for heart rate – Uses Bazett’s Formula or Fridericia’s Formula • Long QT syndrome – inherited or acquired (>75 meds); torsades de pointes/VF; syncope, seizures, sudden death Axis Rhythm • 4 Questions – 1. Are normal P waves present? – 2. Are QRS complexes narrow or wide (≤ or ≥ 0.12)?