Functional Anatomy of Palmar Musculature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study on the Absence of Palmaris Longus in a Multi-Racial Population
108472 NV-OA7 pg26-28.qxd 11/05/2007 05:02 PM Page 26 (Black plate) Malaysian Orthopaedic Journal 2007 Vol 1 No 1 SA Roohi, etal A Study on the Absence of Palmaris Longus in a Multi- racial Population SA Roohi, MS (Ortho) (UKM), L Choon-Sian, MD (UKM), A Shalimar, MS (Ortho) (UKM), GH Tan, MS (Ortho) (UKM), AS Naicker, M Med Rehab (UM) Hospital Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia ABSTRACT Most standard textbooks of hand surgery quote the prevalence of absence of palmaris longus at around 15%3-5. Palmaris longus is a dispensable muscle with a long tendon However, this figure varies considerably in different ethnic which is very useful in reconstructive surgery. It is absent groups. A study by Thompson et al6 on 300 Caucasian 2.8 to 24% of the population depending on the race/ethnicity subjects found that palmaris longus was absent unilaterally in studied. Four hundred and fifty healthy subjects (equally 16%, and bilaterally in 9% of the study sample for an overall distributed among Malaysia’s 3 major ethnic groups) were prevalence of absence of 24%. Similarly, George7 noted on clinically examined for the presence or absence of palmaris 276 cadavers of European descent that its absence was 13% longus. This tendon was found to be absent unilaterally in unilaterally, 8.7% bilaterally for an overall absence of 15.2%. 6.4% of study subjects, and bilaterally in 2.9% of study Another cadaveric study by Vanderhooft8 in Seattle, USA participants. Malays have a high prevalence of palmaris reported its overall absence to be 12%. -

Intrinsic Hand Muscles of the Japanese Monkey, Macaca Fuscata
Anthropol.Sci. 102(Suppl.), 85-95,1994 Intrinsic Hand Muscles of the Japanese Monkey, Macaca fuscata TOSHIHIKO HOMMA AND TATSUO SAKAI Department of Anatomy, School of Medicine, Juntendo University, Hongo 2-1-1, Bunkyo-ku, Tokyo 113, Japan Received December 24, 1993 •ôGH•ô Abstract•ôGS•ô Anatomy of the intrinsic hand muscles in the Japanese monkeys was studied with an improved method to dissect the muscles and nerves in water after removal of the skeletal framework. The thenar eminence contained four muscles, namely m. abductor pollicis brevis, m. opponens pollicis, m, flexor pollicis brevis, and m. adductor pollicis. The hypothenar eminence contained four muscles, namely m. palmaris brevis, m. abductor digiti minimi, m. flexor digiti minimi brevis, and m. opponens digiti minimi. Majority of the thenar muscles are fused more or less with each other, so that clear separation of these muscles was difficult. The lumbrical muscles arose from the palmar parts of four main tendons of the deep flexor muscle to the second, third, fourth, and fifth digits. The mm, contrahentes arose mainly from a medial tendinous septum attached on the palmar surface to the third metacarpus, and included three muscles destined to the second, fourth and fifth digits. The interossei were found on the radial side of the second, third, fourth and fifth finger as well as on the ulnar side of the second, third and fourth finger. The intrinsic hand muscles in the Japanese monkey received innervation either from the median or the ulnar nerve. Branching pattern of these nerves to the individual muscles was fundamentally similar to those in man except for the fact that the median and the ulnar nerve in the Japanese monkey do not communi cateto make a loop in the thenar muscles. -

Pronator Syndrome: Clinical and Electrophysiological Features in Seven Cases
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.5.461 on 1 May 1976. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1976, 39, 461-464 Pronator syndrome: clinical and electrophysiological features in seven cases HAROLD H. MORRIS AND BRUCE H. PETERS From the Department ofNeurology, University of Texas Medical Branch, Galveston, Texas, USA SYNOPSIS The clinical and electrophysiological picture of seven patients with the pronator syndrome is contrasted with other causes ofmedian nerve neuropathy. In general, these patients have tenderness over the pronator teres and weakness of flexor pollicis longus as well as abductor pollicis brevis. Conduction velocity of the median nerve in the proximal forearm is usually slow but the distal latency and sensory nerve action potential at the wrist are normal. Injection of corticosteroids into the pronator teres has produced relief of symptoms in a majority of patients. Protected by copyright. In the majority of isolated median nerve dys- period 101 cases of the carpal tunnel syndrome functions the carpal tunnel syndrome is appropri- and the seven cases of the pronator syndrome ately first suspected. The median nerve can also reported here were identified. Median nerve be entrapped in the forearm giving rise to a conduction velocity determinations were made on similar picture and an erroneous diagnosis. all of these patients. The purpose of this report is to draw full attention to the pronator syndrome and to the REPORT OF CASES features which allow it to be distinguished from Table 1 provides clinical details of seven cases of the median nerve entrapment at other sites. -

Wrist and Hand Examina[On
Wrist and Hand Examinaon Daniel Lueders, MD Assistant Professor Physical Medicine and Rehabilitaon Objecves • Understand the osseous, ligamentous, tendinous, and neural anatomy of the wrist and hand • Outline palpable superficial landmarks in the wrist and hand • Outline evaluaon of and differen.aon between nerves to the wrist and hand • Describe special tes.ng of wrist and hand Wrist Anatomy • Radius • Ulna • Carpal bones Wrist Anatomy • Radius • Ulna • Carpal bones Wrist Anatomy • Radius • Ulna • Carpal bones Wrist Anatomy • Radius • Ulna • Carpal bones Inspec.on • Ecchymosis • Erythema • Deformity • Laceraon Inspec.on • Common Finger Deformies • Swan Neck Deformity • Boutonniere Deformity • Hypertrophic nodules • Heberden’s, Bouchard’s Inspec.on • Swan Neck Deformity • PIP hyperextension, DIP flexion • Pathology is at PIP joint • Insufficiency of volar/palmar plate and suppor.ng structures • Distally, the FDP tendon .ghtens from PIP extension causing secondary DIP flexion • Alternavely, extensor tendon rupture produces similar deformity Inspec.on • Boutonniere Deformity • PIP flexion, DIP hyperextension • Pathology is at PIP joint • Commonly occurs from insufficiency of dorsal and lateral suppor.ng structures at PIP joint • Lateral bands migrate volar/palmar, creang increased flexion moment • Results in PIP “buTon hole” effect dorsally Inspec.on • Nodules • Osteoarthri.c • Hypertrophic changes of OA • PIP - Bouchard’s nodule • DIP - Heberden’s nodule • Rheumatoid Arthri.s • MCP joints affected most • Distal radioulnar joint can also be affected -

Ultrasonograpic Assessment of Relationship Between the Palmaris Longus Tendon and the Flexor Retinacular Ligament and the Palmar Aponeurosis of the Hand
Original Article Ultrasonograpic Assessment of Relationship Between the Palmaris Longus Tendon and the Flexor Retinacular Ligament and the Palmar Aponeurosis of the Hand Kadir Ertem1, Ahmet Sığırcı2, Salih Karaca1, Aykut Sığırcı3, Yunus Karakoç4, Saim Yoloğlu5 İnonu University, Faculty of Medicine, ABSTRACT Departments of Orthopedics and Trauma- tology1, Radioloy2, Physiology4 and Biosta- Aim: This study aimed to evaluate the presence of the Palmaris Longus tistics5, Malatya, Turkey Tendon (PLT) and the relationship between the Flexor Retinacular Ligament (FRL) and the Palmar Aponeurosis (PA) of the hand. 319 Mayıs University, Faculty of Medicine, Departments of Orthopaedics and Trauma- Method: 62 voluntary subjects (31 female, 31 male students and per- tology, Samsun, Turkey sonnel from the Inonu University, at the average age 28.38 ± 6.86 years ranging from 19 to 48 years) took part in this study using ultrasound. Eur J Gen Med 2010;7(2):161-166 Received: 16.05.2009 Result: Significant differences were found in the PA p-m-d diameters of subjects between with and without PLT bilaterally, on the right Accepted: 06.07.2009 and the left hand (p<0.05), whereas there was no meaningful differ- ence considering FRL diameters (p>0.05). Furthermore, this ultraso- nographic assessment revealed the continuity of collagen bunches of the PL tendon up to FRL, but not PA. Conclusion: Although not demonstrated by ultrasonography here, the increased thickness of the PA in subjects with a PLT supports the find- ings in the literature in which the structural -

M1 – Muscled Arm
M1 – Muscled Arm See diagram on next page 1. tendinous junction 38. brachial artery 2. dorsal interosseous muscles of hand 39. humerus 3. radial nerve 40. lateral epicondyle of humerus 4. radial artery 41. tendon of flexor carpi radialis muscle 5. extensor retinaculum 42. median nerve 6. abductor pollicis brevis muscle 43. flexor retinaculum 7. extensor carpi radialis brevis muscle 44. tendon of palmaris longus muscle 8. extensor carpi radialis longus muscle 45. common palmar digital nerves of 9. brachioradialis muscle median nerve 10. brachialis muscle 46. flexor pollicis brevis muscle 11. deltoid muscle 47. adductor pollicis muscle 12. supraspinatus muscle 48. lumbrical muscles of hand 13. scapular spine 49. tendon of flexor digitorium 14. trapezius muscle superficialis muscle 15. infraspinatus muscle 50. superficial transverse metacarpal 16. latissimus dorsi muscle ligament 17. teres major muscle 51. common palmar digital arteries 18. teres minor muscle 52. digital synovial sheath 19. triangular space 53. tendon of flexor digitorum profundus 20. long head of triceps brachii muscle muscle 21. lateral head of triceps brachii muscle 54. annular part of fibrous tendon 22. tendon of triceps brachii muscle sheaths 23. ulnar nerve 55. proper palmar digital nerves of ulnar 24. anconeus muscle nerve 25. medial epicondyle of humerus 56. cruciform part of fibrous tendon 26. olecranon process of ulna sheaths 27. flexor carpi ulnaris muscle 57. superficial palmar arch 28. extensor digitorum muscle of hand 58. abductor digiti minimi muscle of hand 29. extensor carpi ulnaris muscle 59. opponens digiti minimi muscle of 30. tendon of extensor digitorium muscle hand of hand 60. superficial branch of ulnar nerve 31. -
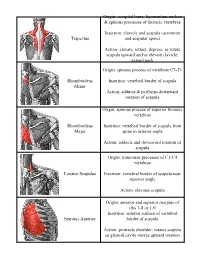
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Anatomy, Shoulder and Upper Limb, Shoulder Muscles
Eovaldi BJ, Varacallo M. Anatomy, Shoulder and Upper Limb, Shoulder Muscles. [Updated 2018 Dec 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534836/ Anatomy, Shoulder and Upper Limb, Shoulder Muscles Authors Benjamin J. Eovaldi1; Matthew Varacallo2. Affilations 1 University of Tennessee HSC 2 Department of Orthopaedic Surgery, University of Kentucky School of Medicine Last Update: December 3, 2018. Introduction The shoulder joint (glenohumeral joint) is a ball and socket joint with the most extensive range of motion in the human body. The muscles of the shoulder dynamically function in performing a wide range of motion, specifically the rotator cuff muscles which function to move the shoulder and arm as well as provide structural integrity to the shoulder joint. The different movements of the shoulder are: abduction, adduction, flexion, extension, internal rotation, and external rotation.[1] The central bony structure of the shoulder is the scapula. All the muscles of the shoulder joint interact with the scapula. At the lateral aspect of the scapula is the articular surface of the glenohumeral joint, the glenoid cavity. The glenoid cavity is peripherally surrounded and reinforced by the glenoid labrum, shoulder joint capsule, supporting ligaments, and the myotendinous attachments of the rotator cuff muscles. The muscles of the shoulder play a critical role in providing stability to the shoulder joint. The primary muscle group that supports the shoulder joint is the rotator cuff muscles. The four rotator cuff muscles include:[2] • Supraspinatus • Infraspinatus • Teres minor • Subscapularis. Structure and Function The upper extremity is attached to the appendicular skeleton by way of the sternoclavicular joint. -

A STUDY of PALMARIS LONGUS MUSCLE: ITS ANATOMIC VARIATIONS with EMBRYOLOGICAL SIGNIFICANCE and CLINICAL IMPORTANCE Sunitha
International Journal of Anatomy and Research, Int J Anat Res 2018, Vol 6(2.2):5222-27. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2018.161 A STUDY OF PALMARIS LONGUS MUSCLE: ITS ANATOMIC VARIATIONS WITH EMBRYOLOGICAL SIGNIFICANCE AND CLINICAL IMPORTANCE Sunitha. R 1, Prathap Kumar J *2. 1 Assistant Professor, Department of Anatomy, Sambhram Institute of Medical Science and Research, KGF, Kolar, Karnataka, India. *2 Assistant Professor, Department of Anatomy, Ramaiah Medical College, Bangalore, Karnataka, India. ABSTRACT Background: In the present study, variations in the Palmaris longus and the clinical implications of these are discussed. Aim: To study the variations in the Palmaris longus and to discuss the embryological basis, clinical and surgical implications of these variations. Materials and Methods: This study was conducted in Department of Anatomy of Hassan Institute of Medical Science, Hassan, Dr B.R.Ambedkar Medical college, Bangalore and Sri Devaraj Urs Academy of Higher Education and Research,Tamaka, Kolar. Thirty formalin fixed cadavers (60 upper limbs); 25 males & 5 female cadavers were dissected for the study and it was conducted over a period of three years, i.e., from 2011-2014. The cadavers with visible trauma, pathology or prior surgeries were excluded from the study. Routine dissection of the upper limb was carried out following the Cunnigham’s Manual of Practical Anatomy. During the dissection of the anterior compartment of forearm, the Palmaris longus muscle was identified & carefully dissected. At first, the origin was confirmed and then, it was traced towards its insertion. Any variations found were noted and photographed. -

Anatomical Study of the Branch of the Palmaris Longus Muscle for Its Transfer to the Posterior Interosseous Nerve
Int. J. Morphol., 37(2):626-631, 2019. Anatomical Study of the Branch of the Palmaris Longus Muscle for its Transfer to the Posterior Interosseous Nerve Estudio Anatómico del Ramo del Músculo Palmar Largo para su Transferencia al Nervio Interóseo Posterior Edie Benedito Caetano1; Luiz Angelo Vieira1; Maurício Benedito Ferreira Caetano2; Cristina Schmitt Cavalheiro3; Marcel Henrique Arcuri3 & Luís Cláudio Nascimento da Silva Júnior3 CAETANO, E. B.; VIEIRA, L. A.; FERREIRA, C. M. B.; CAVALHEIRO, C. S.; ARCURI, M. H. & SILVA JÚNIOR, L. C. N. Anatomical study of the branch of the palmaris longus muscle for its transfer to the posterior interosseous nerve. Int. J. Morphol., 37(2):626-631, 2019. SUMMARY: The objective of the study was to evaluate the anatomical characteristics and variations of the palmaris longus nerve branch and define the feasibility of transferring this branch to the posterior interosseous nerve without tension. Thirty arms from 15 adult male cadavers were dissected after preparation with 20 % glycerin and formaldehyde intra-arterial injection. The palmaris longus muscle (PL) received exclusive innervation of the median nerve in all limbs. In most it was the second muscle of the forearm to be innervated by the median nerve. In 5 limbs the PL muscle was absent. In 5 limbs we identified a branch without sharing branches with other muscles. In 4 limbs it shared origin with the pronator teres (PT), in 8 with the flexor carpi radialis (FCR), in 2 with flexor digitorum superficialis (FDS), in 4 shared branches for the PT and FCR and in two with PT, FCR, FDS. The mean length was (4.0 ± 1.2) and the thickness (1.4 ± 0.6). -

The Muscles That Act on the Upper Limb Fall Into Four Groups
MUSCLES OF THE APPENDICULAR SKELETON UPPER LIMB The muscles that act on the upper limb fall into four groups: those that stabilize the pectoral girdle, those that move the arm, those that move the forearm, and those that move the wrist, hand, and fingers. Muscles Stabilizing Pectoral Girdle (Marieb / Hoehn – Chapter 10; Pgs. 346 – 349; Figure 1) MUSCLE: ORIGIN: INSERTION: INNERVATION: ACTION: ANTERIOR THORAX: anterior surface coracoid process protracts & depresses Pectoralis minor* pectoral nerves of ribs 3 – 5 of scapula scapula medial border rotates scapula Serratus anterior* ribs 1 – 8 long thoracic nerve of scapula laterally inferior surface stabilizes / depresses Subclavius* rib 1 --------------- of clavicle pectoral girdle POSTERIOR THORAX: occipital bone / acromion / spine of stabilizes / elevates / accessory nerve Trapezius* spinous processes scapula; lateral third retracts / rotates (cranial nerve XI) of C7 – T12 of clavicle scapula transverse processes upper medial border elevates / adducts Levator scapulae* dorsal scapular nerve of C1 – C4 of scapula scapula Rhomboids* spinous processes medial border adducts / rotates dorsal scapular nerve (major / minor) of C7 – T5 of scapula scapula * Need to be familiar with on both ADAM and the human cadaver Figure 1: Muscles stabilizing pectoral girdle, posterior and anterior views 2 BI 334 – Advanced Human Anatomy and Physiology Western Oregon University Muscles Moving Arm (Marieb / Hoehn – Chapter 10; Pgs. 350 – 352; Figure 2) MUSCLE: ORIGIN: INSERTION: INNERVATION: ACTION: intertubercular -

Arthroscopic Biceps Tenodesis to Supraspinatus Tendon: Technical Note
Arthroscopic Biceps Tenodesis to Supraspinatus Tendon: Technical Note Laurent Lafosse, MD, Anup A. Shah, MD, Robert B. Butler, MD, and Rachel L. Fowler, BA echniques T & pen or arthroscopic biceps tenodesis is per- In addition, the subscapularis is always thoroughly formed when the proximal biceps tendon is examined. thought to be a pain generator. The biceps Once the decision is made to perform a biceps teno- Otendon generates pain, it is believed, in desis, an additional portal is made through the rotator partial-thickness tears of the biceps, in biceps instability, interval just anterior to the long head of the biceps and in biceps tendinopathy, often occurring with rotator tendon or the supraspinatus (“d” portal in Figure 1B). cuff pathology and affecting shoulder biomechanics. In cases of anterosuperior cuff tears, this area can be echnologies Management of biceps tendon pathology has been a accessed easily from the subacromial space and is often T subject of much interest among shoulder surgeons. used to prepare the tuberosity for cuff repairs. We obtain Several techniques for tenodesis of the biceps tendon this portal with needle localization and use a blunt trocar have been described, but few incorporate the biceps to create a path for instruments and the scope. If no cuff into the rotator cuff tendon. These studies have involved tear is evident, the portal can still be used, but care must techniques that provide a secure tenodesis and fewer be taken not to damage the intact cuff. The lateral portal incisions with good results.1-7 rthopedic A new technique developed by the senior author (L.L.) “Our indications for biceps O incorporates the long head of the biceps tendon with the supraspinatus in patients with anterosuperior rotator cuff tenodesis include biceps tendonitis tears.