Summary of Safety and Effectiveness Data (Ssed)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

42 Pericardiocentesis (Perform) 341
PROCEDURE Pericardiocentesis (Perform) 42 Kathleen M. Cox PURPOSE: Pericardiocentesis is the removal of excess fl uid from the pericardial sac for identifi cation of the etiology of pericardial effusion by fl uid analysis (diagnostic pericardiocentesis) and/or prevention or treatment of cardiac tamponade (therapeutic pericardiocentesis). result of trauma, myocardial infarction, or iatrogenic PREREQUISITE NURSING injury, whereas chronic effusions can result from condi- KNOWLEDGE tions such as bacterial or viral pericarditis, cancer, autoim- mune disorders, uremia, etc. 2 With a decrease in cardiac • Advanced cardiac life support (ACLS) knowledge and output, the patient often develops chest pain, dyspnea, skills are required. tachycardia, tachypnea, pallor, cyanosis, impaired cere- • Knowledge and skills related to sterile technique are bral and renal function, diaphoresis, hypotension, neck needed. vein distention, distant or faint heart sounds, and pulsus • Clinical and technical competence in the performance of paradoxus. 4 pericardiocentesis is required. • The amount of fl uid in the pericardium is evaluated • Knowledge of cardiovascular anatomy and physiology is through chest radiograph, two-dimensional echocardio- needed. gram, electrocardiography (ECG), and clinical fi ndings. • The pericardial space normally contains 20–50 mL of Chest x-rays may not be diagnostically signifi cant in fl uid. patients with acute traumatic tamponade. 6 • Pericardial fl uid has electrolyte and protein profi les similar • Pericardiocentesis to remove fl uid from the pericardial to plasma. sac is performed therapeutically to relieve tamponade or • Pericardial effusion is generally defi ned as the accumula- to diagnose the etiology of the effusion. An acute tampon- tion of fl uid within the pericardial sac that exceeds the ade resulting in hemodynamic instability necessitates an stretch capacity of the pericardium, generally more than emergency procedure. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -
Cryoablation of Pulmonary Veins for the Treatment of Paroxysmal Atrial Fibrillation Coexisting with Isolated Persistent Left
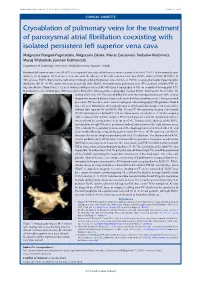
Kardiologia Polska 2018; 76, 11: 1572; DOI: 10.5603/KP.2018.0221 ISSN 0022–9032 CLINICAL VIGNETTE Cryoablation of pulmonary veins for the treatment of paroxysmal atrial fibrillation coexisting with isolated persistent left superior vena cava Małgorzata Peregud-Pogorzelska, Małgorzata Zielska, Marcin Zakrzewski, Radosław Kiedrowicz, Maciej Wielusiński, Jarosław Kaźmierczak Department of Cardiology, Pomeranian Medical University, Szczecin, Poland Persistent left superior vena cava (PLSVC) is a congenital anomaly of the thoracic venous system found in 0.3%–2% of the general popu- lation [1, 2]. In approx. 0.1% of cases, it coexists with the absence of the right superior vena cava (RSVC; isolated PLSVC [IPLSVC]). In 90% of cases PLVSC drains to the right atrium through a dilated coronary sinus (CS) [2, 3]. PLVSC is a potential factor triggering atrial fibrillation (AF) [1, 4]. We report two cases of patients with IPLSVC who underwent pulmonary vein (PV) electrical isolation (PVI) us- ing cryoablation. Case 1 was a 62-year-old man with paroxysmal AF, with typical topography of PVs on computed tomography (CT). Transthoracic echocardiography (TTE) revealed a dilated CS. Intraoperative angiography showed PLVSC draining into the CS (Fig. 1A) A and no RSVC (Fig. 1B). Because of difficulties with the transseptal puncture (TSP), cardiac tamponade occurred but was successfully treated with pericardiocentesis. During a second procedure TSP was done under transoesophageal echocardiography (TEE) guidance. Case 2 was a 69-year-old woman after surgical repair of atrial septal defect type II 20 years earlier, without right superior PV and RSVC (Fig. 1C) on CT. Intraoperative imaging also showed IPLSVC draining into a dilated CS. -

PERICARDIAL ASPIRATION with a NEEDLE ELECTRODE DAVID Jacobso , M.B
• 15 J unie 1963 S.A. TYDSKRIF VIR GENEESKUNDE 637 be stated categorically that UML-491 was solely responsi for further investigation and double-blind trials are sug ble for the apparently good result, because migraine-type gested. headaches are subject to a number of aetiological factors I wish to express my thanks to Dr. P. G. Stein of Sandoz and trigger mechanisms. The paper has been presented for his cooperation in supplying articles and other literature, as a suggestion of how the headache-mechanism cycle as well as for supplying deseril for this trial. might conceivably be broken if the hypothesis that sero tonin is responsible for a number of vascular headaches REFERENCES is acceptable. 1. Graham. J. R. and Wolff. H. G. (1937): Res. Pub!. Assoc. erv. Furthermore, that serotonin antagonism is desirable Ment. Dis.• 181. 63 . in the therapeutic approach to vascular headaches re 2. Wolff. H. G .• Tunis. M. M. and GOOell. H. (1953): Arch. Intern. 3. ~s~~i/~. iZt.: Chapman. R. F .• Godell. H. and·Wolff. H. G. (1957): quires further investigation, as does the 5-HT-headache Psychosom. Med.. 19. 199. relationship. 4. Graham. J. R. (1960): 'ew Eng!. J. Med.• 263. 273. 5. MacGregor. J. MacW. (1963): S. Afr. Med. J .• 37. 168. 6. (a) Wolff. H. G. (1955): Int. Arch. Allergy. 7. 4. SUMMARY (b) Idem (1948): Headache and other Head PaillS. ch. 11. London: Oxford University Press. 1. A short survey of migraine-type vascular headaches 7. Brodie. B. B. et aJ. (1958): Postgrad. Med., 24. 296. 8. Hess. W. R. -

Pericardiocentesis Effective Date: 6 February 2012 (心包穿刺術) Last Review Date: 1 April 2019 Document No.: PILIC0021E Version1.1 Version 1.1 Page 1 of 2
Central Committee on Cardiac Service Pericardiocentesis Effective date: 6 February 2012 (心包穿刺術) Last review date: 1 April 2019 Document no.: PILIC0021E version1.1 Version 1.1 Page 1 of 2 Pericardiocentesis Introduction Collection of fluid in the pericardial space (membranous sac wrapping around the heart) is called pericardial effusion. Causes of pericardial effusion include infection, inflammation, malignancy, metabolic disease, trauma, congestive heart failure, etc. Pericardial effusion can restrict the normal blood filling of the heart and decrease heart function, leading to heart failure. Rapid accumulation of fluid can cause acute pulmonary edema, shock or sudden death. Pericardiocentesis (PC) is an invasive procedure used to treat pericardial effusion. It is done by introducing a drainage tube into the pericardial space, usually under echocardiographic guidance. Importance of Procedure PC serves 2 important purposes. First, by removing abnormal collection of fluid from the pericardial space it restores the normal function of the heart. Second, laboratory tests of the collected fluid can give a diagnosis of the pericardial effusion. Emergency PC is needed in the case of rapid fluid accumulation. If this procedure is refused, the condition of patients can deteriorate rapidly. Alternative treatment methods include surgical opening of the pericardium. The Procedure Echocardiogram is performed to determine the needle entry site. It can be either below the tip of the xiphoid process or at the apex of the heart. The procedural area will be disinfected. Local anesthesia will be given to the needle entry site. A needle is inserted into the pericardial space and a flexible wire introduced through the needle. A hollow tube is exchanged over the wire and secured in the pericardial space. -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

The 2012 Coding Manual Collection
The 2012 Coding Manual Collection SaMple pageS ICD-9-CM Manual, Volumes 1, 2 & 3 (Hospital Edition) ICD-9-CM Manual, Volume 1 & 2 (Professional Edition) HCPCS Level II Manual The following 10 sample pages are from HCpro’s 2011 ICD-9-CM Coding Manual, Volumes 1, 2 & 3 SaMple pageS ICD-9-CM Manual, Volumes 1, 2 & 3 (Hospital Edition) 2011 ICD-9-CM Conventions HCPro’s ICD-9-CM manuals include all the symbols and conventions Please note: Certain categories of codes only consider a particular set of found in the government’s official version (see Preface and ICD-9-CM fifth digits to be a CC condition. Official Guidelines for Coding and Reporting). In addition, HCPro’s ICD- • For example: 9-CM manuals include the following icons and color-coded conventions: Category 403.9x. CC 1 ✓4 TH Fourth digit required. In order to accurately assign a diagnosis This means that ICD-9 code 403.90 is not considered a CC condition, but from this category, it requires the assignment of a fourth digit. 403.91 is designated as a CC condition. • For example: Category 465.x requires a fourth digit of a 0, 8, or 9 to be assigned an accurate code. MCC Major Complication and Comorbidity. This diagnosis is considered a major complication and/comorbidity. Assignment of these ✓5 TH Fifth digit required. In order to accurately assign a diagnosis from diagnoses as an additional code may impact DRG assignment. this category, it requires the assignment of a fifth digit. • For example: 585.6 (End stage renal disease) is • For example: Category 642.0x requires a fifth digit of a 0, 1, 2, 3, or designated as an MCC. -

Pericardial Injury from Chest Compression: a Case Report Of
Aoyagi et al. Journal of Intensive Care (2018) 6:54 https://doi.org/10.1186/s40560-018-0325-5 CASE REPORT Open Access Pericardial injury from chest compression: a case report of incidental release of cardiac tamponade Shigeaki Aoyagi*, Tomokazu Kosuga, Kumiko Wada, Shin-ichi Nata and Hiroshi Yasunaga Abstract Background: Although chest compression is a standard technique in cardiopulmonary resuscitation, it is well recognized that manual chest compression causes various internal injuries, of which major injuries are often fatal. Similarly, when cardiac tamponade occurs in patients with type A acute aortic dissection, many patients die before reaching the hospital. We report a rare case in which chest compressions caused pericardial laceration that may have inadvertently played a life-saving role in releasing cardiac tamponade induced by acute aortic dissection. Case presentation: A 67-year-old woman developed cardiac arrest soon after complaining of epigastric pain, and after successful resuscitation by manual chest compression, she was transferred to our hospital. On arrival, the patient was 14 on the Glasgow Coma Scale. An ECG showed a normal sinus rhythm, and no arrhythmias or signs of myocardial ischemia were observed. A chest X-ray revealed left pleural effusion, while cardiomegaly and pneumothorax were not identified. Computed tomography revealed type A aortic dissection, mild pericardial effusion, and massive left pleural effusion. No pulmonary embolus was found on the CT. After drainage of bloody effusion from the left pleural space, an emergency operation was begun. During surgery, a pericardial laceration with communication to the left pleural space and a hemothorax were found; however, no cardiac injury was identified. -

Fate of the Coronary Ostial and Distal Aortic Anastomoses After Modified Bentall’S Operation (UKC’S Modification)
Chowdhury UK, George N, Singh S, Hasija S, Sankhyan LK, Sharma S, Pandey NN, Sengupta S, Kalaivani M. Fate of the Coronary Ostial and Distal Aortic Anastomoses after Modified Bentall’s Operation (UKC’s Modification). J Surg & Journal of Surgical Tech.2020;2(1):11-20 Surgery and Surgical Technology Original Research Article Open Access Fate of the Coronary Ostial and Distal Aortic Anastomoses after Modified Bentall’s Operation (UKC’s Modification) Ujjwal K. Chowdhury1*, Niwin George1, Sukhjeet Singh1, Suruchi Hasija2, Lakshmikumari Sankhyan1, Srikant Sharma1, Niraj Nirmal Pandey3, Sanjoy Sengupta1, Mani Kalaivani4 1Departments of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, New Delhi, India. 2Department of Cardiac Anaesthesia, All India Institute of Medical Sciences, New Delhi, India. 3Department of Cardiac Radiology ,All India Institute of Medical Sciences, New Delhi, India. 4Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India. Article Info Article Notes Received: May 04, 2020 Accepted: June 13, 2020 *Correspondence: Dr. Ujjwal Kumar Chowdhury, M.Ch, Diplomate NB, Professor, Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029, India; Telephone No: 91-11-26594835; Fax No: 91-11- 26588663, 26588641; Email: [email protected], [email protected]; Orcid ID: http://orcid.org/0000-0002-1672-1502. ©2020 Chowdhury UK. This article is distributed under the terms of the Creative Commons Attribution -

Contrast Echocardiography During Pericardiocentesis
Heart 1999;81:329 329 SHORT CASES IN CARDIOLOGY Heart: first published as 10.1136/hrt.81.3.329 on 1 March 1999. Downloaded from Contrast echocardiography during pericardiocentesis T R Betts, J R Radvan A 35 year old man presented to the casualty department with a two week history of malaise, dyspnoea, and pleuritic chest pain. His tem- perature was 38°C, blood pressure 100/ 75 mm Hg, pulse 105 beats/min and regular, and the heart sounds were quiet. A 12 lead ECG was unremarkable. Chest radiography showed an enlarged cardiac silhouette. Trans- thoracic echocardiography confirmed a 2 cm pericardial eVusion. Pericardiocentesis was performed via the subxiphoid route with the aid of contrast echocardiography (fig 1). Serol- ogy and other investigations suggested a viral aetiology. The catheter was removed after 48 hours and the patient successfully discharged home. Discussion When aspirating pericardial fluid it is impor- tant to be sure that the needle tip is in the peri- http://heart.bmj.com/ cardial space and not a ventricle or pleural cav- ity. DiYculties may arise as perforation into a ventricle does not always produce ventricular ectopic beats or cause chest pain. Additionally, pericardial fluid is often haemorrhagic and may Figure 1 Cross sectional contrast echocardiography, parasternal short axis view, showing pericardial space (PS) be pulsatile. Echocardiographic or fluoroscopic containing a cloud of echogenic microbubbles. (L, left guidance is recommended. Most texts describ- ventricle; N, needle.) ing pericardiocentesis do not detail how echo- cardiography is best used to ensure a safe pro- the precise position of the needle tip in all 16 on September 25, 2021 by guest.