STS Congenital Heart Surgery Database Data Specifications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

Diagnosis and Treatment of Myocarditides
DIAGNOSIS AND TREATMENT OF MYOCARDITIDES Clinical guidelines Task force for preparing the text of recommendations Chairperson: Professor S.N. Tereshchenko (Moscow), Task force members: I.V. Zhirov (MD, Moscow), Professor V.P. Masenko (MD, Moscow), O.Yu. Narusov (Ph.D., Moscow), S.N. Nasonova (Ph.D., Moscow), Professor A.N. Samko (MD, Moscow), O.V. Stukalova (Ph.D., Moscow) and M.A. Shariya (MD, Moscow) Expert Committee: Professor Arutyunov G.P. (Moscow), Professor Moiseev S.V. (Moscow), Professor Vasyuk Yu.A. (Moscow), Professor Garganeeva A.A. (Tomsk), Professor Glezer M.G. (Moscow region), Professor Tkacheva O.N. (Moscow) Professor Shevchenko A.O. (Moscow), Professor Govorin A.V. (Chita), Professor Azizov V.A. (Azerbaijan), Professor Mirrahimov E.M. (Kyrgyzstan), Professor Abdullaev T.A. (Uzbekistan), Ph.D. Panfale E.M. (Moldova), MD Sudzhaeva O.A. (Belarus) Moscow, 2019 TABLE OF CONTENTS Page INTRODUCTION ............................................................................................................. 4 Evidence Base for Diagnosis and Treatment of Myocarditides…………….……6 I. MYOCARDITIDES CLASSIFICATION ................................................................... 5 1. Fulminant myocarditis. .................................................................................................... 6 2. Acute myocarditis. ........................................................................................................... 6 3. Chronic active myocarditis. ............................................................................................ -

Diagnostic Yield and Safety Profile of Endomyocardial Biopsy in the Non
REC Interv Cardiol. 2019;1(2):99-107 Original article Diagnostic yield and safety profile of endomyocardial biopsy in the non-transplant setting at a Spanish referral center Eusebio García-Izquierdo Jaén,a Juan Francisco Oteo Domínguez,a,* Marta Jiménez Blanco,a Cristina Aguilera Agudo,a Fernando Domínguez,a Jorge Toquero Ramos,a Javier Segovia Cubero,a Clara Salas Antón,b Arturo García-Touchard,a José Antonio Fernández-Díaz,a Rodrigo Estévez-Loureiro,a Francisco Javier Goicolea Ruigómez,a and Luis Alonso-Pulpóna a Servicio de Cardiología, Hospital Universitario Puerta de Hierro, Majadahonda, Madrid, Spain b Servicio de Anatomía Patológica, Hospital Universitario Puerta de Hierro, Majadahonda, Madrid, Spain ABSTRACT Introduction and objectives: Endomyocardial biopsy (EMB) is an established diagnostic tool in myocardial disease. However, this technique may carry major complications. We present the diagnostic and safety results of our experience in EMB in the non-trans- plant setting. We also present the results after the implementation of a technical and safety protocol developed at our center. Methods: We retrospectively analyzed the data of all EMBs conducted in non-transplant patients from September 2004 through July 2018. We compared the diagnostic yield and rate of major complications of EMB in two different periods: before and after implementing the protocol. Results: We included 204 EMBs performed in 190 patients. The most frequent indications were the evaluation of ventricular dys- function or suspected myocarditis (51.5%) and the evaluation of restrictive cardiomyopathy or suspected infiltrative disease (44.6%). One hundred and seventy-two EMBs were performed in the right cardiac chambers (84.3%) and 30 EMBs in the left cardiac chambers (14.7%). -

Intractable Chest Pain in Cardiomyopathy: Treatment by a Novel Technique of Cardiac Cryodenervation with Quantitative Immunohistochemical Assessment of Success
574 Br HeartJ 1993;69:574-577 TECHNIQUE Br Heart J: first published as 10.1136/hrt.70.6.574 on 1 December 1993. Downloaded from Intractable chest pain in cardiomyopathy: treatment by a novel technique of cardiac cryodenervation with quantitative immunohistochemical assessment of success J AR Gaer, L Gordon, J Wharton, J M Polak, K M Taylor, W McKenna, D J Parker Abstract she described episodes of chest pain, usually A novel method of cardiac denervation on exertion but also at rest and at night. The by cryoablation has been developed ex- pain occurred up to 10 times daily and her perimentally. The technique uses liquid exercise tolerance had deteriorated from four nitrogen delivered under pressure to miles to 200 yards. Although she denied ablate the principal sources of cardiac paroxysmal nocturnal dyspnoea, she slept innervation-namely, the adventitia sur- with three pillows. Her medication at this rounding the aorta, pulmonary arteries, time consisted of sotalol (80 mg twice daily) and veins. The technique has been veri- and verapamil (240 mg twice daily). She had fied experimentally both in vivo by four children (aged 6, 8, 10, and 11 years) all physiological means and in vitro by of whom were healthy. She was unaware of a quantitative immunohistochemistry and family history of hypertrophic cardiomyopa- the measurement of myocardial nor- thy, although two members of her family had adrenaline concentrations. A 35 year old died suddenly in middle age. On admission a woman presented with intractable pre- soft pansystolic murmur and considerable cordial pain, normal epicardial coronary obesity were noted. -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

Billing and Coding Guidelines Cardiac Catheterization and Coronary Angiography CV-006
Billing and Coding Guidelines LCD Database ID Number L30719 LCD Title Cardiac Catheterization and Coronary Angiography Contractor's Determination Number CV-006 Coding Information General 1. List the appropriate CPT cardiac catheterization code/combination that most clearly describes the service(s) performed. 2. List the appropriate ICD-9 code describing the condition/diagnosis of the patient that is the reason for the right, left, or combined right/left catheterization service(s). 3. Cardiac catheterization codes 93452-93461 include contrast injections, image supervision, interpretation and report for imaging typically performed during these procedures. 4. When injections procedures for right atrial, aortic or pulmonary angiography are performed in conjunction with cardiac catheterization these services (93566-93568) are reported in addition to the appropriate catheterization code. 5. Services considered included in cardiac catheterization/angiography procedures (93452-93461) are as follows, when indicated: a. Local anesthesia and/or sedation b. Introduction, positioning, and repositioning of catheters c. Recording of intracardiac and intravascular pressures d. Obtaining blood samples for blood gases e. Cardiac output measurements f. Monitoring services, e.g., ECCS, arterial pressures, oxygen saturation g. Vascular catheter and line removal h. Final Evaluation i. Written Report 6. Swan Ganz Placement (93503). When a catheter is placed in the right heart for medically necessary monitoring purposes, the code 93503 must be reported. The codes describing a right heart catheterization (e.g., 93451) are used only for medically necessary diagnostic procedures. Do not report code 93503 in conjunction with other diagnostic cardiac catheterization codes. The code 93503 includes: a. Anesthesia or sedation. b. The insertion of the flow-directed catheter. -

Consensus Statement on Endomyocardial Biopsy
Cardiovascular Pathology 21 (2012) 245–274 Original Article 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology ⁎ ⁎ Ornella Leone a, , John P. Veinot b, , Annalisa Angelini c, Ulrik T. Baandrup d, Cristina Basso c, Gerald Berry e, Patrick Bruneval f, Margaret Burke g, Jagdish Butany h, Fiorella Calabrese c, Giulia d'Amati i, William D. Edwards j, John T. Fallon k, Michael C. Fishbein l, Patrick J. Gallagher m, Marc K. Halushka n, Bruce McManus o, Angela Pucci p, E. René Rodriguez q, Jeffrey E. Saffitz r, Mary N. Sheppard g, Charles Steenbergen n, James R. Stone r, Carmela Tan q, Gaetano Thiene c, Allard C. van der Wal s, Gayle L. Winters r aBologna, Italy bOttawa, Ontario, Canada cPadua, Italy dHjoerring, Denmark eStanford, CA, USA fParis, France gLondon, UK hToronto, Ontario, Canada iRome, Italy jRochester, MN, USA kNew York, NY, USA lLos Angeles, CA, USA mSouthampton, UK nBaltimore, MD, USA oVancouver, BC, Canada pPisa, Italy qCleveland, OH, USA rBoston, MA, USA sAmsterdam, The Netherlands Received 3 August 2011; received in revised form 28 September 2011; accepted 7 October 2011 Abstract The Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology have produced this position paper concerning the current role of endomyocardial biopsy (EMB) for the diagnosis of cardiac diseases and its contribution to patient management, focusing on pathological issues, with these aims: • Determining appropriate EMB use in the context of current diagnostic strategies for cardiac diseases and providing recommendations for its rational utilization ⁎ Corresponding authors. O. Leone is to be contacted at U.O. -

Complications of Endomyocardial Biopsy in Heart Transplant Patients: a Retrospective Study of 2117 Consecutive Procedures
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositório Institucional dos Hospitais da Universidade de Coimbra Complications of Endomyocardial Biopsy in Heart Transplant Patients: A Retrospective Study of 2117 Consecutive Procedures F. Saraiva, V. Matos, L. Gonçalves, M. Antunes, and L.A. Providência ABSTRACT Background. Endomyocardial biopsy (EMB) remains the gold standard for the diagno- sis of graft rejection after heart transplantation (HT). Our purpose was to evaluate the rate of complications of this invasive procedure. Methods. This was a retrospective study of 175 patients, who were transplanted between November 2003 and October 2010 and survived more than 1 month after surgery. We evaluated the number of inconclusive EMB and described the incidence, nature, and subsequent management of several complications associated with this procedure. Results. Over a period of approximately 7 years, we performed 2217 EMB yielding 4972 specimens, namely, an average of 2.3 fragments per procedure. The majority of EMBs (95.3%) were performed by the femoral approach. Only 12 EMB (0.57%) were inconclu- sive. The overall complication rate was 0.71%. During puncture, one patient experienced a vasovagal reaction and another one, a femoral artery false aneurysm. During the biopsy, there was one case of cardiac perforation with tamponade, two cases of supraventricular tachycardia, and three atrioventricular conduction abnormalities. In 19 patients, histolog- ical analysis revealed chordal tissue, but only two patients developed mild tricuspid regurgitation. We observed five cases of coronary artery fistulae. The clinical outcomes were favorable in all cases. Conclusion. EMB proved to be a suitable, safe method to monitor rejection after HT. -

42 Pericardiocentesis (Perform) 341
PROCEDURE Pericardiocentesis (Perform) 42 Kathleen M. Cox PURPOSE: Pericardiocentesis is the removal of excess fl uid from the pericardial sac for identifi cation of the etiology of pericardial effusion by fl uid analysis (diagnostic pericardiocentesis) and/or prevention or treatment of cardiac tamponade (therapeutic pericardiocentesis). result of trauma, myocardial infarction, or iatrogenic PREREQUISITE NURSING injury, whereas chronic effusions can result from condi- KNOWLEDGE tions such as bacterial or viral pericarditis, cancer, autoim- mune disorders, uremia, etc. 2 With a decrease in cardiac • Advanced cardiac life support (ACLS) knowledge and output, the patient often develops chest pain, dyspnea, skills are required. tachycardia, tachypnea, pallor, cyanosis, impaired cere- • Knowledge and skills related to sterile technique are bral and renal function, diaphoresis, hypotension, neck needed. vein distention, distant or faint heart sounds, and pulsus • Clinical and technical competence in the performance of paradoxus. 4 pericardiocentesis is required. • The amount of fl uid in the pericardium is evaluated • Knowledge of cardiovascular anatomy and physiology is through chest radiograph, two-dimensional echocardio- needed. gram, electrocardiography (ECG), and clinical fi ndings. • The pericardial space normally contains 20–50 mL of Chest x-rays may not be diagnostically signifi cant in fl uid. patients with acute traumatic tamponade. 6 • Pericardial fl uid has electrolyte and protein profi les similar • Pericardiocentesis to remove fl uid from the pericardial to plasma. sac is performed therapeutically to relieve tamponade or • Pericardial effusion is generally defi ned as the accumula- to diagnose the etiology of the effusion. An acute tampon- tion of fl uid within the pericardial sac that exceeds the ade resulting in hemodynamic instability necessitates an stretch capacity of the pericardium, generally more than emergency procedure. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Outcomes of Mechanical Circulatory Support for Giant Cell Myocarditis: a Systematic Review
Journal of Clinical Medicine Article Outcomes of Mechanical Circulatory Support for Giant Cell Myocarditis: A Systematic Review Preeyal M. Patel 1, Abhiraj Saxena 1, Chelsey T. Wood 1, Thomas J. O’Malley 2 , Elizabeth J. Maynes 2, John W. C. Entwistle 2, H. Todd Massey 2, Preethi R. Pirlamarla 3, René J. Alvarez 3, Leslie T. Cooper 4 , J. Eduardo Rame 2 and Vakhtang Tchantchaleishvili 2,* 1 Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA 19107, USA; [email protected] (P.M.P.); [email protected] (A.S.); [email protected] (C.T.W.) 2 Division of Cardiac Surgery, Department of Surgery, Thomas Jefferson University, Philadelphia, PA 19107, USA; [email protected] (T.J.O.); [email protected] (E.J.M.); john.entwistle@jefferson.edu (J.W.C.E.); howard.massey@jefferson.edu (H.T.M.); eduardo.rame@jefferson.edu (J.E.R.) 3 Division of Cardiology, Department of Medicine, Thomas Jefferson University, Philadelphia, PA 19107, USA; preethi.pirlamarla@jefferson.edu (P.R.P.); rene.alvarez@jefferson.edu (R.J.A.) 4 Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL 32224, USA; [email protected] * Correspondence: vakhtang.tchantchaleishvili@jefferson.edu; Tel.: +1-215-955-6996; Fax: +1-215-955-6010 Received: 20 October 2020; Accepted: 23 November 2020; Published: 1 December 2020 Abstract: Treatment of giant cell myocarditis (GCM) can require bridging to orthotopic heart transplantation (OHT) or recovery with mechanical circulatory support (MCS). Since the roles of MCS and immunotherapy are not well-defined in GCM, we sought to analyze outcomes of patients with GCM who required MCS. -
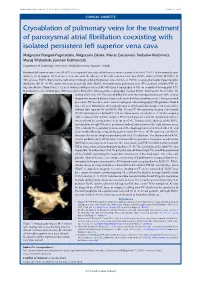
Cryoablation of Pulmonary Veins for the Treatment of Paroxysmal Atrial Fibrillation Coexisting with Isolated Persistent Left
Kardiologia Polska 2018; 76, 11: 1572; DOI: 10.5603/KP.2018.0221 ISSN 0022–9032 CLINICAL VIGNETTE Cryoablation of pulmonary veins for the treatment of paroxysmal atrial fibrillation coexisting with isolated persistent left superior vena cava Małgorzata Peregud-Pogorzelska, Małgorzata Zielska, Marcin Zakrzewski, Radosław Kiedrowicz, Maciej Wielusiński, Jarosław Kaźmierczak Department of Cardiology, Pomeranian Medical University, Szczecin, Poland Persistent left superior vena cava (PLSVC) is a congenital anomaly of the thoracic venous system found in 0.3%–2% of the general popu- lation [1, 2]. In approx. 0.1% of cases, it coexists with the absence of the right superior vena cava (RSVC; isolated PLSVC [IPLSVC]). In 90% of cases PLVSC drains to the right atrium through a dilated coronary sinus (CS) [2, 3]. PLVSC is a potential factor triggering atrial fibrillation (AF) [1, 4]. We report two cases of patients with IPLSVC who underwent pulmonary vein (PV) electrical isolation (PVI) us- ing cryoablation. Case 1 was a 62-year-old man with paroxysmal AF, with typical topography of PVs on computed tomography (CT). Transthoracic echocardiography (TTE) revealed a dilated CS. Intraoperative angiography showed PLVSC draining into the CS (Fig. 1A) A and no RSVC (Fig. 1B). Because of difficulties with the transseptal puncture (TSP), cardiac tamponade occurred but was successfully treated with pericardiocentesis. During a second procedure TSP was done under transoesophageal echocardiography (TEE) guidance. Case 2 was a 69-year-old woman after surgical repair of atrial septal defect type II 20 years earlier, without right superior PV and RSVC (Fig. 1C) on CT. Intraoperative imaging also showed IPLSVC draining into a dilated CS.