Morphological and Enzymatical Characterization of the Infection Process of Pythium Ultimum in Dendrobium Officinale (Orchidaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation of Defense Mechanisms Against Pathogens in Mosses and Flowering Plants
Int. J. Mol. Sci. 2013, 14, 3178-3200; doi:10.3390/ijms14023178 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Activation of Defense Mechanisms against Pathogens in Mosses and Flowering Plants Inés Ponce de León 1,* and Marcos Montesano 2 1 Departamento de Biología Molecular, Instituto de Investigaciones Biológicas Clemente Estable, Avenida Italia 3318, CP 11600, Montevideo, Uruguay 2 Laboratorio de Fisiología Vegetal, Centro de Investigaciones Nucleares, Facultad de Ciencias, Mataojo 2055, CP 11400, Montevideo, Uruguay; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +598-24872605; Fax: +598-24875548. Received: 4 January 2013; in revised form: 23 January 2013 / Accepted: 23 January 2013 / Published: 4 February 2013 Abstract: During evolution, plants have developed mechanisms to cope with and adapt to different types of stress, including microbial infection. Once the stress is sensed, signaling pathways are activated, leading to the induced expression of genes with different roles in defense. Mosses (Bryophytes) are non-vascular plants that diverged from flowering plants more than 450 million years ago, allowing comparative studies of the evolution of defense-related genes and defensive metabolites produced after microbial infection. The ancestral position among land plants, the sequenced genome and the feasibility of generating targeted knock-out mutants by homologous recombination has made the moss Physcomitrella patens an attractive model to perform functional studies of plant genes involved in stress responses. This paper reviews the current knowledge of inducible defense mechanisms in P. patens and compares them to those activated in flowering plants after pathogen assault, including the reinforcement of the cell wall, ROS production, programmed cell death, activation of defense genes and synthesis of secondary metabolites and defense hormones. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School Department of Plant Pathology and Environmental Microbiology CHARACTERIZATION OF Pythium and Phytopythium SPECIES FREQUENTLY FOUND IN IRRIGATION WATER A Thesis in Plant Pathology by Carla E. Lanze © 2015 Carla E. Lanze Submitted in Partial Fulfillment of the Requirement for the Degree of Master of Science August 2015 ii The thesis of Carla E. Lanze was reviewed and approved* by the following Gary W. Moorman Professor of Plant Pathology Thesis Advisor David M. Geiser Professor of Plant Pathology Interim Head of the Department of Plant Pathology and Environmental Microbiology Beth K. Gugino Associate Professor of Plant Pathology Todd C. LaJeunesse Associate Professor of Biology *Signatures are on file in the Graduate School iii ABSTRACT Some Pythium and Phytopythium species are problematic greenhouse crop pathogens. This project aimed to determine if pathogenic Pythium species are harbored in greenhouse recycled irrigation water tanks and to determine the ecology of the Pythium species found in these tanks. In previous research, an extensive water survey was performed on the recycled irrigation water tanks of two commercial greenhouses in Pennsylvania that experience frequent poinsettia crop loss due to Pythium aphanidermatum. In that work, only a preliminary identification of the baited species was made. Here, detailed analyses of the isolates were conducted. The Pythium and Phytopythium species recovered during the survey by baiting the water were identified and assessed for pathogenicity in lab and greenhouse experiments. The Pythium species found during the tank surveys were: a species genetically very similar to P. sp. nov. OOMYA1702-08 in Clade B2, two distinct species of unknown identity in Clade E2, P. -

University of Dundee Genome Sequence of the Necrotrophic Plant
University of Dundee Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire Levesque, C. Andre; Brouwer, Henk; Cano, Liliana; Hamilton, John P.; Holt, Carson; Huitema, Edgar Published in: Genome Biology DOI: 10.1186/gb-2010-11-7-r73 Publication date: 2010 Licence: CC BY Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Levesque, C. A., Brouwer, H., Cano, L., Hamilton, J. P., Holt, C., Huitema, E., Raffaele, S., Robideau, G. P., Thines, M., Win, J., Zerillo, M. M., Beakes, G. W., Boore, J. L., Busam, D., Dumas, B., Ferriera, S., Fuerstenberg, S. I., Gachon, C. M. M., Gaulin, E., ... Buell, C. R. (2010). Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biology, 11(7), -. [R73]. https://doi.org/10.1186/gb-2010-11-7-r73 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Epidemiology of Pythium Sulcatum Associated with Brown-Blotted Root Rot of Carrots
日 植 病 報 62: 130-133 (1996) Ann. Phytopathol. Soc. Jpn. 62: 130-133 (1996) Epidemiology of Pythium sulcatum Associated with Brown-blotted Root Rot of Carrots Koji KAGEYAMA*, Misako TACHI*, Masakazu UMETSU* and Mitsuro HYAKUMACHI* Abstract Pythium sulcatum was a predominant species isolated from carrot lesions showing brown-blotted root rot, and induced the similar symptoms to those naturally occurring in carrot fields with artificial inoculation. The fungus was also isolated from asymptomatic storage and absorbing roots from seedling to harvest stage, especially during spring cropping season. The infection sites of P. sulcatum was the upper part of asymptomatic storage roots in which the blotted lesion might appear. P. ultimum, P. sylvaticum, P. coloratum and P. spinosum were also obtained from asymptomatic roots and residues of root or leaf, but these species showed slight pathogenicity. The absorbing root residue was found to be the primary infection source of P. sulcatum. P. sulcatum was widespread in the intensive carrot-cultivated area. (Received September 20, 1995; Accepted December 27, 1995) Key words: carrot, Pythium sulcatum, brown-blotted root rot, epidemiology. lesions were cut into ca. 5mm blocks, directly plated on INTRODUCTION the Pythium selective medium4) (cornmeal agar (CMA) amended with 5mg/l pimaricin, 100mg/l pentachloro- Brown-blotted root rot of carrot (Daucus carota L.) nitrobenzene and 100mg/l agrimycin), and incubated at first occurred in a main carrot-producing area of Chiba 25•Ž in the dark for 3-7 days. Prefecture9), and later in Kakamigahara of Gifu Prefec- Carrot materials together with storage and absorbing ture in which carrots have been cropped twice in a year. -
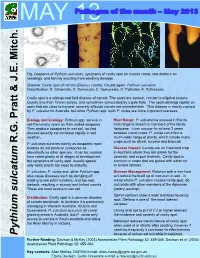
Pythium Sulcatum, Symptoms of Cavity Spot on Mature Carrot, Root Dieback on Seedlings, and Forking Resulting from Seedling Damage
MAY 13Pathogen of the month – May 2013 Fig. Oospores of Pythium sulcatum, symptoms of cavity spot on mature carrot, root dieback on seedlings, and forking resulting from seedling damage. Disease: Cavity spot of carrots (Daucus carota); Causal agent: Pythium sulcatum Classification: K: Chromista, D: Oomycota, C: Oomycetes, O: Pythiales, F: Pythiaceae Cavity spot is a widespread field disease of carrots. The spots are sunken, circular to elliptical lesions usually less than 10 mm across, and sometimes surrounded by a pale halo. The spots develop rapidly on roots that are close to harvest; severely affected carrots are unmarketable. This disease is mainly caused by P. sulcatum in Australia, but other Pythium spp. such P. violae are more important overseas. Biology and Ecology: Pythium spp. survive in Host Range: P. sulcatum is unusual in that its soil from many years as thick walled oospores. host range is limited to members of the family They produce zoospores in wet soil, so that Apiaceae, it can survive for at least 2 years disease severity can increase rapidly in wet between carrot crops. P. violae can infect a weather. much wider range of plants, which include many R.G. Pratt & J.E. Mitch. & J.E. R.G. Pratt crops such as wheat, lucerne and broccoli. P. sulcatum survives mainly as oospores; most isolates do not produce zoospores as Disease Impact: Carrots are an important crop abundantly as other species. It can be isolated in Australia where they are grown for the from carrot plants at all stages of development, domestic and export markets. Cavity spot is but symptoms of cavity spot usually appear common in crops that are grown with either no only when plants are close to maturity. -

Microbial Suppression of Pythium Root Rot in Soilless Systems
Microbial Suppression of Pythium Root Rot in Soilless Systems Cora McGehee B.S. Louisiana State University, 2015 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science At the University of Connecticut 2018 Copyright by Cora Shields McGehee 2018 ii APPROVAL PAGE Master Thesis Microbial Suppression of Pythium Root Rot in Soilless Systems Presented by Cora Shields McGehee, B.S. Major Advisor_________________________________________________________________ Rosa E. Raudales Associate Advisor______________________________________________________________ Wade H. Elmer Associate Advisor_______________________________________________________________ Richard J. McAvoy University of Connecticut 2018 iii Acknowledgements I am beyond grateful to my major advisor Dr. Rosa Raudales for her hard work and dedication to the lab and innovative research projects. Her guidance and patience has made me into a stronger researcher and diligent worker. I also want to thank the other members of my committee Dr. Wade Elmer and Dr. Richard McAvoy for their time and generous advice. I want to thank Frederick Pettit, Shelley Durocher, and Ronald Brine for their assistance in the various greenhouse projects conducted. Thank you Margery Daughtrey for supplying isolates for experiments. Special thanks to Juan Cabrera, Sohan Aziz, Steve Olenski, Joy Tosakoon, and Carla Caballero for giving your time to help with experiments. Lastly a thank you to the office staff, Christine Strand and Nicole Gabelman for their assistance and kindness. Special thanks to the U.S. Department of Agriculture via the Connecticut Department of Agriculture Specialty Crop Block Grant # AG151260 for its support and funding of this work. Thank you to friends and family who supported me over the phone during this intense intellectual pursuit. -

Carrot Cavity Spot
Factsheet 03/03 Horticultural Bradbourne House Development East Malling Council Kent ME19 6DZ Carrots T: 01732 848383 F: 01732 848498 Project No. FV 5a–f E: [email protected] Carrot cavity spot By Tim Pettitt, HRI Wellesbourne and Peter Gladders, ADAS Cavity spot of carrot, caused by slow-growing Pythium species, is currently the most economically important disease problem in UK carrot crops. Affected carrots, with only one or two visible lesions, are rejected at grading. When disease incidence in the field passes a relatively low threshold it becomes uneconomical to harvest crops. This means that virtually 100% control is necessary to avoid economic losses. In a severe cavity spot season, 15–20% or more of crops can be rejected by pack-houses. This amounts to an estimated loss of £10.2 –13.6 million based on the 2000–2001 value of the crop (DEFRA Horticultural Statistics, 2001). Although good control of the disease was achieved in the early 1980s with metalaxyl and related fungicides, there have recently been increasing problems in the management of cavity spot. This factsheet summarises recent HDC funded research and its application for management of the disease. Symptoms of cavity spot The appearance of cavities normally starts as small, pale, sunken elliptical spots, under an apparently intact outer skin. These lesions gradually darken in colour to a greyish brown and, depending on environmental conditions (see below), they may increase in size rapidly, or expand more slowly with the natural growth of the carrot root. Eventually, the outer skin ruptures, leaving an open cavity where the tissues underneath have been attacked by the fungus and secondary organisms. -

Management of Damping-Off Caused by Pythium Spp. in Organic
MANAGEMENT OF DAMPING-OFF CAUSED BY PYTHIUM SPP. IN ORGANIC VEGETABLE PRODUCTION IN THE PACIFIC NORTHWEST By ANA VIDA CRISOSTOMO ALCALA A thesis submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Plant Pathology JULY 2013 To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of ANA VIDA CRISOSTOMO ALCALA find it satisfactory and recommend that it be accepted. __________________________________ Lindsey du Toit, Ph.D., Chair __________________________________ Carol Miles, Ph.D. __________________________________ Tim Paulitz, Ph.D. __________________________________ Lyndon Porter, Ph.D. ii ACKNOWLEDGEMENTS Professional I am greatly indebted to four great scientists who mentored me in my PhD. My deepest gratitude is to Dr. Lindsey du Toit, my major advisor, for opening the door of opportunity for me to pursue my Ph.D. in the US, and for providing me with every chance to develop a variety of skills essential for this profession. Her enthusiasm, passion, and dedication to research have been inspirational to me. To my committee members, Dr. Carol Miles, Dr. Tim Paulitz, and Dr. Lyndon Porter, who generously took time to mentor me, by distance or whenever I got a chance to work on campus, as well as for generously sharing their expertise. To the very hardworking VSP crew: Mike, Barb, Sarah, John, and Anita, for all their help in one or many aspects of this project. Particularly to Mike, for his expertise and tremendous help in my field, greenhouse, and lab trials; Barb, for always being ready to help even beyond the call of duty, and for always making what seemed an impossible task possible. -

University of Florida Thesis Or Dissertation Formatting
INNOVATIVE TOOLS FOR STUDYING PLANT-RELATED OOMYCETE POPULATIONS OF POTENTIAL AND CURRENT THREAT IN AGRICULTURE IN ECUADOR By MARIA FERNANDA RATTI TORRES A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Maria Fernanda Ratti Torres To my Obi-Wan Roberto, Yoda one for me. You have been nothing but supportive during these years, you make me proud of being your wife, but above all, you make me immensely happy. This is for you and for a marriage that has been put to rest for so long and it is ready to resume. ACKNOWLEDGMENTS I wish to thank my parents for all their effort and the patience, they are my pillar without whom I could not have pursued my Ph.D. studies. Also to my siblings Andrea and Pablo, my brother in law Carlos and my nephews, not only for their support, but for joking around all the time and cheering me up during this journey. Erica M. Goss deserves a special section only for her, but the formatting will not allow it. I cannot imagine having spent these years under anybody else’s guidance. She always challenged me to be better, to be calmed during stressful situations and to trust in myself. Her advices will be forever in my mind. Doing this research would have been impossible without helping hands around the world: Thanks to Esther Lilia P. for all her support, to Juan C., Carlos A., Jerry L. -

Final Report
Final Report Effective Management of Parsley Summer Root Rot Project leader: Dr Len Tesoriero Delivery partner: NSW Department of Primary Industries Project code: VG13101 Hort Innovation – Final Report Project: Effective Management of Parsley Summer Root Rot – VG13101 Disclaimer: Horticulture Innovation Australia Limited (Hort Innovation) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. Hort Innovation is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from Hort Innovation or any other person’s negligence or otherwise) from your use or non‐use of the Final Report or from reliance on information contained in the Final Report or that Hort Innovation provides to you by any other means. Funding statement: This project has been funded by Hort Innovation, using the vegetable research and development levy, co‐ investment from NSW Department of Primary Industries and contributions from the Australian Government. Hort Innovation is the grower‐owned, not‐for‐profit research and development corporation for Australian horticulture. Publishing details: ISBN 978 0 7341 4454 -

Using Genomic Data to Understand Anthropogenic Influences on Oomycete and Phytophthora Communities, and the Evolution of an Alie
USING GENOMIC DATA TO UNDERSTAND ANTHROPOGENIC INFLUENCES ON OOMYCETE AND PHYTOPHTHORA COMMUNITIES, AND THE EVOLUTION OF AN ALIEN INVASIVE SPECIES RESPONSIBLE FOR SUDDEN OAK DEATH, PHYTOPHTHORA RAMORUM. by ANGELA DALE A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Forestry) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) April 2018 © Angela Dale, 2018 Abstract Emerging Phytophthora pathogens, often introduced, represent a threat to natural ecosystems. Phytophthora species are known for rapid adaptation and hybridization, which may be facilitated by anthropogenic activities. Little is known about natural Phytophthora and oomycete populations, or mechanisms behind rapid adaptation. We surveyed oomycete and Phytophthora communities from southwest B.C. under varying anthropogenic influences (urban, interface, natural) to determine effects on diversity, introductions and migration. We used DNA meta- barcoding to address these questions on oomycetes. We then focused on Phytophthora, adding baiting and culturing methods, and further sub-dividing urban sites into agricultural or residential. Finally, we studied an alien invasive species, Phytophthora ramorum responsible for sudden oak death, and how it overcame the invasion paradox, limited to asexual reproduction and presumed reduced adaptability. Anthropogenic activities increase oomycete and Phytophthora diversity. Putative introduced species and hybrids were more frequent in urban sites. Migration is suggested by shared species between urban and interface sites, and two known invasive species found in natural and interface sites. Different anthropogenic activities influence different communities. Abundance increased for some species in either residential or agricultural sites. Two hybrids appear to be spreading in different agricultural sites. -
A Desk Study to Review Global Knowledge on Best Practice for Oomycete Root-Rot Detection and Control
Project title: A desk study to review global knowledge on best practice for oomycete root-rot detection and control Project number: CP 126 Project leader: Dr Tim Pettitt Report: Final report, March 2015 Previous report: None Key staff: Dr G M McPherson Dr Alison Wakeham Location of project: University of Worcester Stockbridge Technology Centre Industry Representative: Russ Woodcock, Bordon Hill Nurseries Ltd, Bordon Hill, Stratford-upon-Avon, Warwickshire, CV37 9RY Date project commenced: April 2014 Date project completed April 2015 AHDB Horticulture is a Division of the Agriculture and Horticulture Development Board DISCLAIMER While the Agriculture and Horticulture Development Board seeks to ensure that the information contained within this document is accurate at the time of printing, no warranty is given in respect thereof and, to the maximum extent permitted by law the Agriculture and Horticulture Development Board accepts no liability for loss, damage or injury howsoever caused (including that caused by negligence) or suffered directly or indirectly in relation to information and opinions contained in or omitted from this document. ©Agriculture and Horticulture Development Board 2015. No part of this publication may be reproduced in any material form (including by photocopy or storage in any medium by electronic mean) or any copy or adaptation stored, published or distributed (by physical, electronic or other means) without prior permission in writing of the Agriculture and Horticulture Development Board, other than by reproduction in an unmodified form for the sole purpose of use as an information resource when the Agriculture and Horticulture Development Board or AHDB Horticulture is clearly acknowledged as the source, or in accordance with the provisions of the Copyright, Designs and Patents Act 1988.