Carrot Cavity Spot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytopythium: Molecular Phylogeny and Systematics
Persoonia 34, 2015: 25–39 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158515X685382 Phytopythium: molecular phylogeny and systematics A.W.A.M. de Cock1, A.M. Lodhi2, T.L. Rintoul 3, K. Bala 3, G.P. Robideau3, Z. Gloria Abad4, M.D. Coffey 5, S. Shahzad 6, C.A. Lévesque 3 Key words Abstract The genus Phytopythium (Peronosporales) has been described, but a complete circumscription has not yet been presented. In the present paper we provide molecular-based evidence that members of Pythium COI clade K as described by Lévesque & de Cock (2004) belong to Phytopythium. Maximum likelihood and Bayesian LSU phylogenetic analysis of the nuclear ribosomal DNA (LSU and SSU) and mitochondrial DNA cytochrome oxidase Oomycetes subunit 1 (COI) as well as statistical analyses of pairwise distances strongly support the status of Phytopythium as Oomycota a separate phylogenetic entity. Phytopythium is morphologically intermediate between the genera Phytophthora Peronosporales and Pythium. It is unique in having papillate, internally proliferating sporangia and cylindrical or lobate antheridia. Phytopythium The formal transfer of clade K species to Phytopythium and a comparison with morphologically similar species of Pythiales the genera Pythium and Phytophthora is presented. A new species is described, Phytopythium mirpurense. SSU Article info Received: 28 January 2014; Accepted: 27 September 2014; Published: 30 October 2014. INTRODUCTION establish which species belong to clade K and to make new taxonomic combinations for these species. To achieve this The genus Pythium as defined by Pringsheim in 1858 was goal, phylogenies based on nuclear LSU rRNA (28S), SSU divided by Lévesque & de Cock (2004) into 11 clades based rRNA (18S) and mitochondrial DNA cytochrome oxidase1 (COI) on molecular systematic analyses. -

Activation of Defense Mechanisms Against Pathogens in Mosses and Flowering Plants
Int. J. Mol. Sci. 2013, 14, 3178-3200; doi:10.3390/ijms14023178 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Activation of Defense Mechanisms against Pathogens in Mosses and Flowering Plants Inés Ponce de León 1,* and Marcos Montesano 2 1 Departamento de Biología Molecular, Instituto de Investigaciones Biológicas Clemente Estable, Avenida Italia 3318, CP 11600, Montevideo, Uruguay 2 Laboratorio de Fisiología Vegetal, Centro de Investigaciones Nucleares, Facultad de Ciencias, Mataojo 2055, CP 11400, Montevideo, Uruguay; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +598-24872605; Fax: +598-24875548. Received: 4 January 2013; in revised form: 23 January 2013 / Accepted: 23 January 2013 / Published: 4 February 2013 Abstract: During evolution, plants have developed mechanisms to cope with and adapt to different types of stress, including microbial infection. Once the stress is sensed, signaling pathways are activated, leading to the induced expression of genes with different roles in defense. Mosses (Bryophytes) are non-vascular plants that diverged from flowering plants more than 450 million years ago, allowing comparative studies of the evolution of defense-related genes and defensive metabolites produced after microbial infection. The ancestral position among land plants, the sequenced genome and the feasibility of generating targeted knock-out mutants by homologous recombination has made the moss Physcomitrella patens an attractive model to perform functional studies of plant genes involved in stress responses. This paper reviews the current knowledge of inducible defense mechanisms in P. patens and compares them to those activated in flowering plants after pathogen assault, including the reinforcement of the cell wall, ROS production, programmed cell death, activation of defense genes and synthesis of secondary metabolites and defense hormones. -

U.S. EPA, Pesticides, Label, V-10161 4 SC, 4/20/2011
--- -..:.--~--- ~- ~- >- --=---==-- -"--====- c· ott! 2£lll C '" . zo( UNITED STATES ENVI~ONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFElY AND POLLUTION PREVENTION APR 2 0 20H Robert Hamilton Valent USA Corporation Registration & Regulatory Affairs 1101 14th Street, N.W., Suite 1050 Washington, DC 20005 SUBJECT: Label Amendment V-101614SC EPA Reg. No. 59639-140; Decisions 409896; 420444; 9F7617 (D420455) Submissions Dated April 30, 2009; September 16, 2009 Dear Mr. Hamilton: The revised master and supplemental labels (your version 3/15/2011) referred to above, submi~ed in connection with registration under the Federal Insecticide Fungicide and Rodenticide Act (FIFRA), as amended, to add carrot, potato, and sugarbeet which, with existing crops allows listing of the entire "Root and Tuber Vegetables-Crop Group 1"; and which adds "Brassica, Leafy Greens Subgroup 5B" which, with existing crops allows listing the entire "Bras sica (Cole) Leafy Vegetables, Crop Group 5", all of this in detail as per final rule published 4/20/2011, are acceptable provided the following label changes and conditional data are satisfied ' by specified due dates: 1. At the top of page 1 delete the right and left parentheses from "(Fungicide)" because the rest of this label does the same and we understand the primary brand name to be the "V-10161 4SC Fungicide"; also add a comma after "(Except Brassica Vegetables)," and on page 2 in the First Aid section in the subheading "If on skin or Clothing", make the "C" in "Clothing" lower case and add a period at the end of the last bullet. 2. On page 3 in the Agricultural Use Requirements box, first line; add "(WPS)" after "Worker Protection Standard". -

Pythium Species Associated with Rooibos, and the Influence of Management Practices on Disease Development
PYTHIUM SPECIES ASSOCIATED WITH ROOIBOS, AND THE INFLUENCE OF MANAGEMENT PRACTICES ON DISEASE DEVELOPMENT By AMIRHOSSEIN BAHRAMISHARIF Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in the Faculty of AgriSciences at the University of Stellenbosch Supervisor: Dr. Adéle McLeod Co-supervisor: Dr. Sandra C. Lamprecht March 2012 Stellenbosch University http://scholar.sun.ac.za DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Amirhossein Bahramisharif Date:……………………….. Copyright © 2012 Stellenbosch University All rights reserved Stellenbosch University http://scholar.sun.ac.za PYTHIUM SPECIES ASSOCIATED WITH ROOIBOS, AND THE INFLUENCE OF MANAGEMENT PRACTICES ON DISEASE DEVELOPMENT SUMMARY Damping-off of rooibos (Aspalathus linearis), which is an important indigenous crop in South Africa, causes serious losses in rooibos nurseries and is caused by a complex of pathogens of which oomycetes, mainly Pythium, are an important component. The management of damping-off in organic rooibos nurseries is problematic, since phenylamide fungicides may not be used. Therefore, alternative management strategies such as rotation crops, compost and biological control agents, must be investigated. The management of damping-off requires knowledge, which currently is lacking, of the Pythium species involved, and their pathogenicity towards rooibos and two nursery rotation crops (lupin and oats). Pythium species identification can be difficult since the genus is complex and consists of more than 120 species. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School Department of Plant Pathology and Environmental Microbiology CHARACTERIZATION OF Pythium and Phytopythium SPECIES FREQUENTLY FOUND IN IRRIGATION WATER A Thesis in Plant Pathology by Carla E. Lanze © 2015 Carla E. Lanze Submitted in Partial Fulfillment of the Requirement for the Degree of Master of Science August 2015 ii The thesis of Carla E. Lanze was reviewed and approved* by the following Gary W. Moorman Professor of Plant Pathology Thesis Advisor David M. Geiser Professor of Plant Pathology Interim Head of the Department of Plant Pathology and Environmental Microbiology Beth K. Gugino Associate Professor of Plant Pathology Todd C. LaJeunesse Associate Professor of Biology *Signatures are on file in the Graduate School iii ABSTRACT Some Pythium and Phytopythium species are problematic greenhouse crop pathogens. This project aimed to determine if pathogenic Pythium species are harbored in greenhouse recycled irrigation water tanks and to determine the ecology of the Pythium species found in these tanks. In previous research, an extensive water survey was performed on the recycled irrigation water tanks of two commercial greenhouses in Pennsylvania that experience frequent poinsettia crop loss due to Pythium aphanidermatum. In that work, only a preliminary identification of the baited species was made. Here, detailed analyses of the isolates were conducted. The Pythium and Phytopythium species recovered during the survey by baiting the water were identified and assessed for pathogenicity in lab and greenhouse experiments. The Pythium species found during the tank surveys were: a species genetically very similar to P. sp. nov. OOMYA1702-08 in Clade B2, two distinct species of unknown identity in Clade E2, P. -

University of Dundee Genome Sequence of the Necrotrophic Plant
University of Dundee Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire Levesque, C. Andre; Brouwer, Henk; Cano, Liliana; Hamilton, John P.; Holt, Carson; Huitema, Edgar Published in: Genome Biology DOI: 10.1186/gb-2010-11-7-r73 Publication date: 2010 Licence: CC BY Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Levesque, C. A., Brouwer, H., Cano, L., Hamilton, J. P., Holt, C., Huitema, E., Raffaele, S., Robideau, G. P., Thines, M., Win, J., Zerillo, M. M., Beakes, G. W., Boore, J. L., Busam, D., Dumas, B., Ferriera, S., Fuerstenberg, S. I., Gachon, C. M. M., Gaulin, E., ... Buell, C. R. (2010). Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biology, 11(7), -. [R73]. https://doi.org/10.1186/gb-2010-11-7-r73 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Epidemiology of Pythium Sulcatum Associated with Brown-Blotted Root Rot of Carrots
日 植 病 報 62: 130-133 (1996) Ann. Phytopathol. Soc. Jpn. 62: 130-133 (1996) Epidemiology of Pythium sulcatum Associated with Brown-blotted Root Rot of Carrots Koji KAGEYAMA*, Misako TACHI*, Masakazu UMETSU* and Mitsuro HYAKUMACHI* Abstract Pythium sulcatum was a predominant species isolated from carrot lesions showing brown-blotted root rot, and induced the similar symptoms to those naturally occurring in carrot fields with artificial inoculation. The fungus was also isolated from asymptomatic storage and absorbing roots from seedling to harvest stage, especially during spring cropping season. The infection sites of P. sulcatum was the upper part of asymptomatic storage roots in which the blotted lesion might appear. P. ultimum, P. sylvaticum, P. coloratum and P. spinosum were also obtained from asymptomatic roots and residues of root or leaf, but these species showed slight pathogenicity. The absorbing root residue was found to be the primary infection source of P. sulcatum. P. sulcatum was widespread in the intensive carrot-cultivated area. (Received September 20, 1995; Accepted December 27, 1995) Key words: carrot, Pythium sulcatum, brown-blotted root rot, epidemiology. lesions were cut into ca. 5mm blocks, directly plated on INTRODUCTION the Pythium selective medium4) (cornmeal agar (CMA) amended with 5mg/l pimaricin, 100mg/l pentachloro- Brown-blotted root rot of carrot (Daucus carota L.) nitrobenzene and 100mg/l agrimycin), and incubated at first occurred in a main carrot-producing area of Chiba 25•Ž in the dark for 3-7 days. Prefecture9), and later in Kakamigahara of Gifu Prefec- Carrot materials together with storage and absorbing ture in which carrots have been cropped twice in a year. -
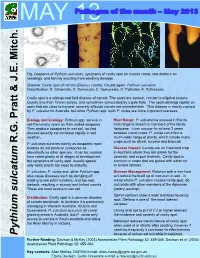
Pythium Sulcatum, Symptoms of Cavity Spot on Mature Carrot, Root Dieback on Seedlings, and Forking Resulting from Seedling Damage
MAY 13Pathogen of the month – May 2013 Fig. Oospores of Pythium sulcatum, symptoms of cavity spot on mature carrot, root dieback on seedlings, and forking resulting from seedling damage. Disease: Cavity spot of carrots (Daucus carota); Causal agent: Pythium sulcatum Classification: K: Chromista, D: Oomycota, C: Oomycetes, O: Pythiales, F: Pythiaceae Cavity spot is a widespread field disease of carrots. The spots are sunken, circular to elliptical lesions usually less than 10 mm across, and sometimes surrounded by a pale halo. The spots develop rapidly on roots that are close to harvest; severely affected carrots are unmarketable. This disease is mainly caused by P. sulcatum in Australia, but other Pythium spp. such P. violae are more important overseas. Biology and Ecology: Pythium spp. survive in Host Range: P. sulcatum is unusual in that its soil from many years as thick walled oospores. host range is limited to members of the family They produce zoospores in wet soil, so that Apiaceae, it can survive for at least 2 years disease severity can increase rapidly in wet between carrot crops. P. violae can infect a weather. much wider range of plants, which include many R.G. Pratt & J.E. Mitch. & J.E. R.G. Pratt crops such as wheat, lucerne and broccoli. P. sulcatum survives mainly as oospores; most isolates do not produce zoospores as Disease Impact: Carrots are an important crop abundantly as other species. It can be isolated in Australia where they are grown for the from carrot plants at all stages of development, domestic and export markets. Cavity spot is but symptoms of cavity spot usually appear common in crops that are grown with either no only when plants are close to maturity. -

Microbial Suppression of Pythium Root Rot in Soilless Systems
Microbial Suppression of Pythium Root Rot in Soilless Systems Cora McGehee B.S. Louisiana State University, 2015 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science At the University of Connecticut 2018 Copyright by Cora Shields McGehee 2018 ii APPROVAL PAGE Master Thesis Microbial Suppression of Pythium Root Rot in Soilless Systems Presented by Cora Shields McGehee, B.S. Major Advisor_________________________________________________________________ Rosa E. Raudales Associate Advisor______________________________________________________________ Wade H. Elmer Associate Advisor_______________________________________________________________ Richard J. McAvoy University of Connecticut 2018 iii Acknowledgements I am beyond grateful to my major advisor Dr. Rosa Raudales for her hard work and dedication to the lab and innovative research projects. Her guidance and patience has made me into a stronger researcher and diligent worker. I also want to thank the other members of my committee Dr. Wade Elmer and Dr. Richard McAvoy for their time and generous advice. I want to thank Frederick Pettit, Shelley Durocher, and Ronald Brine for their assistance in the various greenhouse projects conducted. Thank you Margery Daughtrey for supplying isolates for experiments. Special thanks to Juan Cabrera, Sohan Aziz, Steve Olenski, Joy Tosakoon, and Carla Caballero for giving your time to help with experiments. Lastly a thank you to the office staff, Christine Strand and Nicole Gabelman for their assistance and kindness. Special thanks to the U.S. Department of Agriculture via the Connecticut Department of Agriculture Specialty Crop Block Grant # AG151260 for its support and funding of this work. Thank you to friends and family who supported me over the phone during this intense intellectual pursuit. -

Management of Damping-Off Caused by Pythium Spp. in Organic
MANAGEMENT OF DAMPING-OFF CAUSED BY PYTHIUM SPP. IN ORGANIC VEGETABLE PRODUCTION IN THE PACIFIC NORTHWEST By ANA VIDA CRISOSTOMO ALCALA A thesis submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Plant Pathology JULY 2013 To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of ANA VIDA CRISOSTOMO ALCALA find it satisfactory and recommend that it be accepted. __________________________________ Lindsey du Toit, Ph.D., Chair __________________________________ Carol Miles, Ph.D. __________________________________ Tim Paulitz, Ph.D. __________________________________ Lyndon Porter, Ph.D. ii ACKNOWLEDGEMENTS Professional I am greatly indebted to four great scientists who mentored me in my PhD. My deepest gratitude is to Dr. Lindsey du Toit, my major advisor, for opening the door of opportunity for me to pursue my Ph.D. in the US, and for providing me with every chance to develop a variety of skills essential for this profession. Her enthusiasm, passion, and dedication to research have been inspirational to me. To my committee members, Dr. Carol Miles, Dr. Tim Paulitz, and Dr. Lyndon Porter, who generously took time to mentor me, by distance or whenever I got a chance to work on campus, as well as for generously sharing their expertise. To the very hardworking VSP crew: Mike, Barb, Sarah, John, and Anita, for all their help in one or many aspects of this project. Particularly to Mike, for his expertise and tremendous help in my field, greenhouse, and lab trials; Barb, for always being ready to help even beyond the call of duty, and for always making what seemed an impossible task possible. -

University of Florida Thesis Or Dissertation Formatting
INNOVATIVE TOOLS FOR STUDYING PLANT-RELATED OOMYCETE POPULATIONS OF POTENTIAL AND CURRENT THREAT IN AGRICULTURE IN ECUADOR By MARIA FERNANDA RATTI TORRES A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Maria Fernanda Ratti Torres To my Obi-Wan Roberto, Yoda one for me. You have been nothing but supportive during these years, you make me proud of being your wife, but above all, you make me immensely happy. This is for you and for a marriage that has been put to rest for so long and it is ready to resume. ACKNOWLEDGMENTS I wish to thank my parents for all their effort and the patience, they are my pillar without whom I could not have pursued my Ph.D. studies. Also to my siblings Andrea and Pablo, my brother in law Carlos and my nephews, not only for their support, but for joking around all the time and cheering me up during this journey. Erica M. Goss deserves a special section only for her, but the formatting will not allow it. I cannot imagine having spent these years under anybody else’s guidance. She always challenged me to be better, to be calmed during stressful situations and to trust in myself. Her advices will be forever in my mind. Doing this research would have been impossible without helping hands around the world: Thanks to Esther Lilia P. for all her support, to Juan C., Carlos A., Jerry L. -

Final Report
Final Report Effective Management of Parsley Summer Root Rot Project leader: Dr Len Tesoriero Delivery partner: NSW Department of Primary Industries Project code: VG13101 Hort Innovation – Final Report Project: Effective Management of Parsley Summer Root Rot – VG13101 Disclaimer: Horticulture Innovation Australia Limited (Hort Innovation) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. Hort Innovation is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from Hort Innovation or any other person’s negligence or otherwise) from your use or non‐use of the Final Report or from reliance on information contained in the Final Report or that Hort Innovation provides to you by any other means. Funding statement: This project has been funded by Hort Innovation, using the vegetable research and development levy, co‐ investment from NSW Department of Primary Industries and contributions from the Australian Government. Hort Innovation is the grower‐owned, not‐for‐profit research and development corporation for Australian horticulture. Publishing details: ISBN 978 0 7341 4454