Fourth Annual Report on Project SHARE’S Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spotted Trout Or Landlocked Salmon INFORMATION FREE
VOL. XXVII. NO. 52. PHILLIPS, MAINE, FRIDAY, AUGUST 4, 1905. PRICE 3 CENTS. JSPORTSMBN^S—SUPPLIES | SPORTSMEN’S SUPPLIES Fish and Game Oddities. SPORTSMEN'S S UPPLIE S SPORTSMEN’S SUPPLIES One of the mail carriers report seeing near the “ county bridge” in Madrid, a fox carrying a cat in it’s mouth. The fox seemed surprised at the meeting, stood still for an instant then clashed RIFLE AND PISTOL CARTF. DGES into the woods, still holding the cat. j Winchester Rifle and Pistol Cartridges of Several years ago the writer saw a fox catch a young crow. To escape the all calibers are loaded by machinery which pursuing crows ihe fox stampeded some sizes the shells, supplies the exact quantity METALLIC CARTRIDGES colts in the pasture and kept with them of powder, and seats the bullets properly. until near a thicket of small trees, then By using first-class materials and this Old and enthusiastic hunters who have “ tried them ail,” use U. M. C, Cart disappeared leaving the crows to talk ridges and recommend them to their friends. up-to-date system of loading, the reputation Ko matter what make or model of rifle you use, —U. M. C. Cartridges will give the matter over by themselves. jperior results. Buy just the right Cartridges for your gun—U. M. C. Cart of Winchester Cartridges for accuracy, Iges. Every dealer—City or country—sells U. M. C. Fish Don’t A l l Suit Them. ’"eliability and excellence is maintained. Use Cartridges made hy Cartridge specialists, 17, M . C. -

River Herring Program – 2009‐2016 Grants
River Herring Program – 2009‐2016 Grants RIVER HERRING PROGRAM River Herring NORTHEAST REGION Assessing Sustainability of Maine River Herring Runs Maine Department of Marine Resources Maine Award Amount....................................................................... $400,483 Grantee Match ...................................................................... $415,340 Total Project ............................................................................ $815,823 Collect river herring population data on numerous rivers in order to create management and harvest models to help ensure the sustainability of the fishery. Project will also hire an education specialist to work with inland communities to help gain acceptance for reintroduction of river herring. River Herring Bycatch Avoidance in Small Mesh Fisheries (MA) University of Massachusetts Massachusetts Award Amount....................................................................... $305,640 Grantee Match ...................................................................... $376,929 Total Project ............................................................................ $682,569 Develop river herring bycatch avoidance incentive systems based on models that identify and predict high concentrations of river herring. Project will help to minimize bycatch of river herring in the Atlantic herring and mackerel fisheries in New England. Updated May 2017 River Herring Program – 2009‐2016 Grants Identification and Modeling of Alewife Stock Structure Gulf of Maine Research Institute -

Brook Floater (Alasmidonta Varicosa) in the West River in Vermont
Brook Floater (Alasmidonta varicosa) in the West River in Vermont prepared for Vermont Fish and Wildlife Wildlife Diversity Program Montpelier, Vermont prepared by biodrawversity Biodrawversity LLC 206 Pratt Corner Road, Leverett, Massachusetts December 2014 a Brook Floater (Alasmidonta varicosa) in the West River in Vermont The West River in Newfane, Vermont, at quantitative monitoring site 2 (Q-2). INTRODUCTION the Brook Floater population could withstand such high mortality. The 2002 survey docu mented startlingly low The Brook Floater (Alasmidonta varicosa) is one of the numbers of Brook Floater (alive or dead) compared to most imperiled freshwater animals in northeastern the early 1990s (Ferguson 2002). North America. It is listed as Endangered in Massachu- setts, New Hampshire, and Connecticut; Threatened In 2008, Ethan Nedeau conducted qualitative and quan- in Vermont and Maine; and extirpated in Rhode Island titative freshwater mussel sur veys in the West River in (Nedeau 2008). The West River in southeastern Vermont Townshend and Newfane (Biodrawversity 2008). The holds Ver mont’s only Brook Floater population (Fichtel main objectives were to assess the status of the Brook and Smith 1995, Biodrawversity 2008). The species was Floater at the Green Bridge Pool and Scotts Covered discovered in the West River in 1979-1980, and has since Bridge, compare results to prior surveys, and present a been found sporadically from Jamaica to Brattleboro, clearer picture of the viability of the Brook Floater popu- usually at low population densities. High-density popu- lation. Six species were encountered during the survey, lations were documented downstream of the Townsh- end Dam, a flood control dam operated by the U.S. -

Ibastoryspring08.Pdf
irds find Maine attractive for many of the same reasons we do—the state offers a unique blend of landscapes spanning from mountains to the sea, with forests, grasslands, rivers, marshes, and long coastlines in between. B Where we find beautiful places to hike and kayak, camp and relax, birds find the habitat they need for their survival. But while Maine’s diverse habitats serve an important role for over IBAs 400 bird species—some threatened, endangered, or of regional conservation in concern—the state’s not immune to a growing list of threats that puts these birds at further risk. Habitat loss, degradation, and fragmentation due to development, toxins such as mercury and lead, oil spills on the coast and Maine inland waters, and climate change are top among them. BY ANDREW COLVIN In the face of these threats, a crucial step in conserving Maine’s birds is to identify the areas of the state that are most important for breeding, wintering, and migration. After several years of working toward that goal, Maine Audubon Lists Maine Audubon has recently completed the first phase of its Important 22 of the Most Important Bird Areas (IBA) program, identifying 22 areas across Maine that are vital Places in Maine for Vulnerable Birds to state—and even global—bird populations. HANS TOOM ERIC HYNES Eight of the rare birds used to identify IBAs in Maine (clockwise from left): Short-eared owl, black-throated blue warbler, least tern, common moorhen, scarlet tanager, harlequin duck, saltmarsh sharp-tailed sparrow, and razorbill. MIKE FAHEY Important -

IMPORTANT BIRD AREAS of MAINE an Analysis Of
IMPORTANT BIRD AREAS OF MAINE An Analysis of Avian Diversity and Abundance Compiled by: Susan Gallo, Thomas P. Hodgman, and Judy Camuso A Project Supported by the Maine Outdoor Heritage Fund IMPORTANT BIRD AREAS OF MAINE An Analysis of Avian Diversity and Abundance February 7, 2008 Compiled by: Susan Gallo, Maine Audubon, 20 Gilsland Farm Rd., Falmouth, ME 04105 Thomas P. Hodgman, Maine Department of Inland Fisheries and Wildlife, 650 State St., Bangor, ME 04401 Judy Camuso, Maine Audubon, 20 Gilsland Farm Rd., Falmouth, ME 04105 (Present Address: Maine Department of Inland Fisheries and Wildlife, 358 Shaker Road, Gray, ME 04039) Recommended citation: Gallo, S., T. P. Hodgman, and J. Camuso, Compilers. 2008. Important Bird Areas Of Maine: an analysis of avian diversity and abundance. Maine Audubon, Falmouth, Maine. 94pp. Cover Photo: Scarborough Marsh at sunrise, by W. G. Shriver ii Table of Contents History ..........................................................................................................................................1 What is an Important Bird Area?.......................................................................................1 Qualifying Criteria...................................................................................................................1 Data Use and Applicability Disclaimer .............................................................................2 Acknowledgements...................................................................................................................3 -

Alasmidonta Varicosa) Version 1.1.1
Species Status Assessment Report for the Brook Floater (Alasmidonta varicosa) Version 1.1.1 Molunkus Stream, Tributary of the Mattawamkeag River in Maine. Photo credit: Ethan Nedeau, Biodrawversity. Inset: Adult brook floaters. Photo credit: Jason Mays, USFWS. July 2018 U.S. Fish and Wildlife Service This document was prepared by Sandra Doran of the New York Ecological Services Field Office with assistance from the U.S. Fish and Wildlife Service Brook Floater Species Status Assessment (SSA) Team. The team members include Colleen Fahey, Project Manager (Species Assessment Team (SAT), Headquarters (HQ) and Rebecca Migala, Assistant Project Manager, (Region 1, Regional Office), Krishna Gifford (Region 5, Regional Office), Susan (Amanda) Bossie (Region 5 Solicitor's Office, Julie Devers (Region 5, Maryland Fish and Wildlife Conservation Office), Jason Mays (Region 4, Asheville Field Office), Rachel Mair (Region 5, Harrison Lake National Fish Hatchery), Robert Anderson and Brian Scofield (Region 5, Pennsylvania Field Office), Morgan Wolf (Region 4, Charleston, SC), Lindsay Stevenson (Region 5, Regional Office), Nicole Rankin (Region 4, Regional Office) and Sarah McRae (Region 4, Raleigh, NC Field Office). We also received assistance from David Smith of the U.S. Geological Survey, who served as our SSA Coach. Finally, we greatly appreciate our partners from Department of Fisheries and Oceans, Canada, the Brook Floater Working Group, and others working on brook floater conservation. Version 1.0 (June 2018) of this report was available for selected peer and partner review and comment. Version 1.1 incorporated comments received on V 1.0 and was used for the Recommendation Team meeting. This final version, (1.1.1), incorporates additional comments in addition to other minor editorial changes including clarifications. -
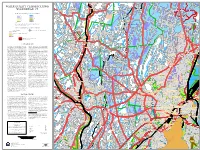
Water Quality Classification
t d S Simpson Lake R t d Cammerino Dam No 1 s d S s Exit 24 R g d C r g P R w g n R L o i r n A a a D o n ed d l l i Laurel Ledge School C y l Res No 3 o l o t R i R r n r r n J d i d i t d i r d d o ge R k C m n Rid r c o o p utum s r R A D t n a W H n R a i o Downs Road Pond r d u a g o ! L w e y s S C n i k a d D e e k r n n B T o Lander Pond Towantic Brook g s n Rd n o 9 H d e no m r R e a d i eb l a L W A L L I N G F O R D d A r a i r u B 6 L r L s o d R n T R r H a y D n D D L Butterworth Pond t C R y F r y k t w w a e a G r k u i m n e n a e l O y o in AA r e n r H lo g i o a o r t t b t m d on W y r A d r H M D D hitn u s e y o r e A y r u a t un Ct q R R s e Res No 2 w ta d h a D E M in S h t B ta t B d P t a R B r an erkins d r o Odd Pond S x m Rd d o o y n a S k k x e C P N n d sv e p o R A a e r d rr le E t o a c y d h w A v n r s P o R e U Chestnut Hill p R d o n pso n n D r r Ln L d R R e r n Butterworth Brook u d L a R d d e a n D C Fairwood Pond L W ATER Q UALITY CLAS S IFICATIONS a n Sheldon Dr n Mill River o o w s h e o D l d n W o l o l ilson R s p Dr d C a B Beacon Falls Station Hockanum Brook W n a i i rc B n w e N D W h s J i o l w B s R a o o r M u L o f n t F d n i so u o 4 D o A u n u r A n r 3 R r d r AA u d e a n s t e a T J ! h d e 6 r p à S M l R e Munson Road Pond s o n t F d M i e o To o A d Sanford Brook z y m r o v F s e t S r n o w d y d d L u a D am a e R x l P d o D w p r R e R w D R 67 Marian Ln E R d a iv C c n M e v H R d h O k l S A r r V R l z y l n R t etha d l i d Butterworth Dam B p i r A a -

Discover New Places to Hike, Bike
Allagash Falls by Garrett Conover Explore MAINE 2019 WHAT’S INSIDE: Discover New Places to Hike, Bike, Swim, & More Favorite Protected Places Where in Maine do you want to go this summer? This year’s edition of Explore Maine offers spectacular places personally picked by NRCM staff, board, and members who know them well. Working together, over the last Books & Blogs 60 years, we helped ensure these places would be always be protected, for generations to come. We hope by NRCM Members you’ll make time to enjoy any and all of these recommendations. For even more ideas, visit our online Explore Maine map at www.nrcm.org. Cool Apps It is also our pleasure to introduce you to books and blogs by NRCM members. Adventure books, Explore Great Maine Beer biographies, children’s books, poetry—this year’s collection represents a wonderful diversity that you’re sure to enjoy. Hear first-hand from someone who has taken advantage of the discount many Maine sporting camps Maine Master provide to NRCM members. Check out our new map of breweries who are members of our Maine Brewshed Naturalist Program Alliance, where you can raise a glass in support of the clean water that is so important for great beer. And Finding Paradise we’ve reviewed some cool apps that can help you get out and explore Maine. Enjoy, and thank you for all you do to help keep Maine special. Lots More! —Allison Wells, Editor, Senior Director of Public Affairs and Communications Show your love for Explore Maine with NRCM a clean, beautiful Paddling, hiking, wildlife watching, cross-country skiing—we enjoy spending time in Maine’s great outdoors, and you’re invited to join us! environment Find out what’s coming up at www.nrcm.org. -

NEFMC EFH Desigations
NEFMC EFH Desigations developed as part of Omnibus Essential Fish Habitat Amendment 2 Amendment 14 to the Northeast Multispecies FMP Amendment 14 to the Atlantic Sea Scallop FMP Amendment 4 to the Monkfish FMP Amendment 3 to the Atlantic Herring FMP Amendment 2 to the Red Crab FMP Amendment 2 to the Skate FMP Amendment 3 to the Atlantic Salmon FMP New England Fishery Management Council 50 Water Street, Mill 2 Newburyport, MA 01950 (978) 465-0492 tel. Essential Fish Habitat or EFH is define as those waters necessary for spawning, breeding, feeding, and growth to maturity. Regional Fishery Management Councils are required to desginate EFH per the 1996 reauthorization of the Magnuson Stevens Fishery Conservation and Management Act. Regulatory guidance about EFH designations and EFH consultations was published in 2002 by the National Oceanic and Atmospheric Administration’s National Marine Fisheries Service (Federal Register, Vol. 67, No. 12, p 2343-2383). This guidance recommends description and identification of EFH by species and lifestage, based on the best available sources of information. Per the guidance, both text descriptions of essential habitats as well as spatial depictions of the extent of EFH should be developed. The New England Fishery Management Council developed its current EFH designations via Omnibus Habitat Amendment 2 (OHA2). OHA2 represented the first update to the NEFMC’s original EFH designations, developed in 1999 or shortly thereafter. Development of OHA2 began in 2004, and the final regulations were implemented on April 9, 2018. The EFH designations were the primary focus of the first phase of work on the amendment, from 2004- 2007, but adjustments to the desginations were made throughout the process, up until final Council action in April and June of 2016. -

Atlantic Salmon EFH the Proposed EFH Designation for Atlantic Salmon
Atlantic salmon EFH The proposed EFH designation for Atlantic salmon includes the rivers, estuaries, and bays that are listed in Table 31 and shown in Map 105, which exhibit the environmental conditions defined in the text descriptions. Smaller tributaries not shown on the map are also EFH for one or more life stage as long as they conform to the proposed habitat descriptions. All EFH river systems form a direct connection to the sea, but EFH would not include portions of rivers above naturally occurring barriers to upstream migration or land-locked lakes and ponds. The oceanic component of EFH is to a distance of three miles from the mouth of each river. The new designation includes six new drainage systems not included in the original list of 26 rivers that were designated in 1998. All of them are in the Maine coastal sub-region (Chandler, Indian, Pleasant, St. George, Medomak, and Pemaquid rivers). All told, 30 river systems in nine New England sub-regions are designated for Atlantic salmon EFH. The new map includes a more continuous series of bays and areas adjacent to river mouths that are within three miles of the coast. Designated EFH in Long Island Sound has been reduced to small areas where the Connecticut and Pawcatuck Rivers empty into the sound, rather than taking up the entire sound. Also, there are a number of improvements in the text descriptions which make the habitat requirements for each life stage more specific and applicable to three separate juvenile life stages (fry, parr, and smolts). Text descriptions: Essential fish habitat for Atlantic salmon (Salmo salar) is designated as the rivers, estuaries, and bays that are listed in Table 31 and shown in Map 105. -

THE FLOODS of MARCH 1936 Part 1
If you do jno*-Be <l this report after it has served your purpose, please return it to the Geolocical -"" Survey, using the official mailing label at the end UNITED STATES DEPARTMENT OF THE INTERIOR THE FLOODS OF MARCH 1936 Part 1. NEW ENGLAND RIVERS Prepared in cooperation withihe FEDERAL EMERGENCY ADMINISTRATION OF PUBLIC WORKS GEOLOGICAL SURVEY WATER-SUPPLY PAPER 798 UNITED STATES DEPARTMENT OF THE INTERIOR Harold L. Ickes, Secretary GEOLOGICAL SURVEY W. C. Mendenhall, Director Water-Supply Paper 798 THS^LOODS OF MARCH 1936 PART 1. NEW ENGLAND RIVERS NATHAN C. GROVER Chief Hydraulic Engineer Prepared in cooperation with the FEDERAL EMERGENCY ADMINISTRATION OF PUBLIC WORKS UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1937 For sale by the Superintendent of Documents, Washington, D. C. Price 70 cents CONTENTS Page Abstract............................................................. 1 Introduction......................................................... 2 Authorization........................................................ 5 Administration and personnel......................................... 5 Acknowledgments...................................................... 6 General features of the storms....................................... 7 Floods of the New England rivers....................................o 12 Meteorologic and hydrologic conditions............................... 25 Precipitation records............................................ 25 General f>!-................................................... 25 Distr<* '-utlon -

C S a S S C C S
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Proceedings Series 2004/046 Série des comptes rendus 2004/046 Mitigation for Acid Rain Impacts on Atténuation des effets des pluies Atlantic Salmon and Their Habitat acides sur le saumon atlantique et son habitat 19-20 April 2004 Les 19 et 20 avril 2004 Atlantic Salmon Federation Centre de conférences de la Conference Center Fédération du saumon atlantique Chamcook, New Brunswick Chamcook, Nouveau-Brunswick T.L. Marshall1 (Chair), D. Kircheis2 T.L. Marshall1 (Chair), D. Kircheis2 T. Clair3, and K.A. Rutherford1 T. Clair3, and K.A. Rutherford1 1 1 Department of Fisheries and Oceans Ministère des Pêches et des Océans P.O. Box 1006, Dartmouth, N.S. B2Y 4A2 C. P. 1006, Dartmouth (N.-É.) B2Y 4A2 2National Oceanic and Atmospheric 2National Oceanic and Atmospheric Administration (NOAA) Administration (NOAA) 17 Godfrey Drive, Orono, ME 04473 17 Godfrey Drive, Orono, ME 04473 3 Environment Canada 3 Environnement Canada P.O. Box 6227, Sackville, N.B. E4L 1G6 C. P. 6227, Sackville (N.-B.) E4L 1G6 May 2005 / mai 2005 Foreword The purpose of these proceedings is to archive the activities and discussions of the meeting, including research recommendations, uncertainties, and to provide a place to formally archive official minority opinions. As such, interpretations and opinions presented in this report may be factually incorrect or mis-leading, but are included to record as faithfully as possible what transpired at the meeting. No statements are to be taken as reflecting the consensus of the meeting unless they are clearly identified as such.