SYNTHESIS, SPECTROSCOPIC and BIOLOGICAL STUDIES of 3D TRANSITION METAL COMPLEXES with VARIOUS ANILINE BASED SCHIFF BASES HABIB H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001
CONFERENCE REPORT 732 CHIMIA 2001, 55, No.9 CONFERENCE REPORT Chimia 55 (2001) 732-736 © Schweizerische Chemische Gesellschaft ISSN 0009-4293 Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001 Christian G. Bochet* Keywords: CERC3 . Champery . European chemistry' Organic synthesis· Workshop The chairmen of the European Re- from the chiral centers, had a strong im- The day was concluded with the first search Councils' Chemistry Committees pact on the geometry of the dimeric cata- plenary lecture, by Professor Varinder (CERC3) elected organic synthesis as lyst; these effects could be so strong that Aggarwal (University of Bristol, UK). this year's topic for their annual work- similar catalysts varying only by one sub- He explained the asymmetric epoxida- shop, and Switzerland as the host coun- stituent gave opposite enantiomers [1]. tion reaction, in which, instead of using try. The conference chairman, Prof. The afternoon session was concluded the classical strategy (i.e. oxidation of an Philippe Renaud made the excellent by Petri Pihko (The Scripps Research In- alkene), the key step is a carbon-carbon choice of Champery for hosting the stitute, La Jolla, USA), on the synthesis bond forming process (the so-called Co- workshop; the combination of his enthu- of Azaspiracid (Fig. 1), a very potent tox- rey-Chaykovsky epoxidation). The beau- siasm, efficient organization and the in, isolated from Irish mussels. The FGHI ty of this reaction, very simple in its ex- beautiful alpine scenery made this meet- spirocyclic portion proved to be a very perimental realization, is revealed by the ing a really unique event. -
XIII Spring Meeting
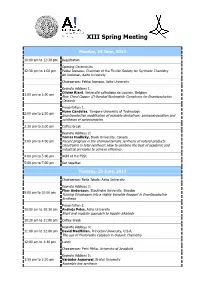
XIII Spring Meeting Monday, 24 June, 2013 10:00 am to 12:30 pm Registration Opening Ceremonies 12:30 pm to 1:00 pm Pekka Joensuu, Chairman of the Finnish Society for Synthetic Chemistry Ari Koskinen, Aalto University Chairperson: Pekka Joensuu, Aalto University Keynote Address 1: Olivier Riant, Université catholique de Louvain, Belgium 1:00 pm to 2:00 pm New Chiral Copper (I)-Bonded Nucleophile Complexes for Enantioselective Catalysis Presentation 1: Nuno Candeias, Tampere University of Technology 2:00 pm to 2:30 pm Enantioselective modification of oxindole derivatives: aminooxygenation and syntheses of spirooxindoles 2:30 pm to 3:00 pm Coffee break Keynote Address 2: Tomas Hudlicky, Brock University, Canada 3:00 pm to 4:00 pm Recent progress in the chemoenzymatic synthesis of natural products. Constraints in total synthesis: How to combine the best of academic and industrial principles to achieve efficiency. 4:00 pm to 5:00 pm AGM of the FSSC 5:00 pm to 7:00 pm Get together Tuesday, 25 June, 2013 Chairperson: Reija Jokela, Aalto University Keynote Address 3: Pher Andersson, Stockholm University, Sweden 9:00 am to 10:00 am Turning Dihydrogen into a Highly Versatile Reagent in Enantioselective Synthesis Presentation 2: 10:00 am to 10:30 am Andrejs Pelss, Aalto University Short and modular approach to lepadin alkaloids 10:30 am to 11:00 am Coffee Break Keynote Address 4: 11:00 am to 12:00 am David MacMillan, Princeton University, U.S.A. The use of Photoredox Catalysis in Organic Chemistry 12:00 am to 1:30 pm Lunch Chairperson: Petri Pihko, -

FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr
FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr. Lee D. Arnold 1987, Ph.D. Serine β-Lactones in Syntheses of Amino Acids None President & CEO of Discovery Elucidations [email protected] Dr. Hanaa Assil 1989, M.Sc. Solid Supports for Azodicarboxylates in Mitsunobu Reactions n/a Unknown Unknown and Synthesis of Amino Acids Dr. Karine Auclair 1999, Ph.D. Biosynthetic Studies on the Polyketide Lovastatin: Enzyme- Prof. P. Ortiz De Montellano, Department Assoc. Professor, Department of Chemistry, McGill University, [email protected] Catalyzed Diels-Alder Reactions of Pharmaceutical Chemistry, University of Montreal, Que., Canada California at San Francisco, USA Dr. Jennifer Blunston (nee Caplan) 2001, Ph.D. Diaminopimelic Acid Analogues as Inhibitors of Enzymes Prof. D. Waisman, Departments of Stay-at-home mom, raising son Rhys. Previously Jennifer [email protected] Involved in Bacterial Lysine Biosynthesis Biochemistry and Oncology, University of Blunston was Assistant Professor of Chemistry, St. Mary's Calgary, Canada University College, Calgary, AB, Canada Dr. Marc Boudreau 2007, Ph.D. Nucleoside Dicarboxylates as Mimics of Diphosphates and Prof. Hagan Bayley, Oxford University Postdoc with Professor Shahriar Mobashery, University of Notre [email protected] Disulfide Bond Replacement in Pediocin PA1 Dame Dr. Doug Burr 2006, Ph.D. Studies of the in vitro Activity of Lovastatin Nonaketide Prof. David Sherman, Dept. Medicinal Research scientist at Perkin Elmer, Boston, Massachusetts [email protected] Synthase Chemistry, University of Michigan, Ann Arbor, MI Dr. Hengmiao (Henry) Cheng 1992, Ph.D. Mechanism and Inhibition of Peptidylglycine α-Hydroxlating Prof. E.J. Corey, Department of Chemistry, Senior Research Investigator, Pfizer Global Research and [email protected] Monooxygenase Harvard University Development, Pfizer Inc., Groton, CT, USA Dr. -

Abstracts for ICP2019
The 29th International Conference on Photochemistry Boulder, Colorado • July 21 – 26, 2019 ABSTRACTS The 29th International Conference on Photochemistry Boulder, Colorado July 21 – 26, 2019 PLENARY SPEAKER ABSTRACTS PLENARY PRESENTATIONS ICP2019 Code: Plenary Presenter: Hiroshi MASUHARA (TWN) Abstract Title: Optical Manipulation in Chemistry Co-Author(s): Abstract: Photochemistry and spectroscopy study various dynamics and mechanism of molecules and materials induced by their interaction with light. Optical force is another interaction between light and matter, which was experimentally confirmed by Lebedev in late 19th century. Utilizing this force Ashkin proposed as “Optical Tweezers” in 1986 and demonstrated high potential in application to bio-science, which was awarded Nobel Prize Physics last year. Optical force was also expected to control molecular motion in solution and eventually chemical reaction. In 1988 we started to explore new molecular phenomena characteristic of optical force by combining optical trapping with fluorescence, absorption, electrochemical, and ablation methods. We have studied on microparticles, microdroplets, nanoparticles, polymers, supramolecules, micelles, amino acids and proteins in solution and reported their unique behavior. The remarkable achievement was performed in 2007 when the trapping laser was focused at solution surface. Various amino acids are crystalized, giving one single crystal at the position where trapping laser is focused and at the time when irradiated. This optical manipulation at solution surface is extended to dielectric and gold nanoparticles and to glass/solution interface. The initially trapped polystyrene nanoparticles at the focus form their periodical structure and scatter/propagate the trapping laser, shifting trapping site outside of the focus and forming a single large circle assembly. -

Tim JONCKERS
1 ST ALPINE WINTER CONFERENCE ON MEDICINAL AND SYNTHETIC CHEMISTRYTim ST. ANTON, AUSTRIA | JANUARYJONCKERS 28 - FEBRUARY 1, 2018 JANSSEN PHARMACEUTICA Prof. Erick BOOK OF ABSTRACTS CONTENT SPEAKERS & ORAL COMMUNICATIONS - Biographies and Abstracts 3 POSTERS - Addressing Preclinical Toxicity – Approaches and Lessons Learned 70 POSTERS - Advances in Lead Generation 72 POSTERS - Advances in Synthetic Methods 75 POSTERS - Alternative Modalities 95 POSTERS - Challenges and Opportunities in Fragment Based Drug Discovery 98 POSTERS - Chemical Biology in Drug and Target Discovery 104 POSTERS - Drug Discovery Tales 121 POSTERS - Late Stage Functionalization 137 LIST OF ABSTRACTS 139 LIST OF AUTHORS 145 - 2 - SPEAKERS & ORAL COMMUNICATIONS Biographies and Abstracts - 3 - Erick M. CARREIRA ETH Zürich, Switzerland rof. Erick M. Carreira obtained a B.S. degree in 1984 from the University of Illinois at Urbana- PChampaign under the supervision of Scott E. Denmark and a Ph.D. degree in 1990 from Harvard University under the supervision of David A. Evans. After carrying out postdoctoral work with Peter Dervan at the California Institute of Technology through late 1992, he joined the faculty at the same institution as an assistant professor of chemistry and subsequently was promoted to the rank of associate professor in the Spring of 1996, and full professor in Spring 1997. Since September 1998, he has been professor of chemistry at the ETH Zürich in the Institute of Organic Chemistry. In 2011, he became associated with the Competence Center for Systems Physiology and Metabolic Diseases at ETH-Zürich. e is the recipient of numerous awards. Professor Carreira’s interests encompass several facets Hof chemical synthesis: natural products synthesis, chemistry as well as biology, catalysis, medicinal chemistry, and synthetic methods. -

Biologically Active Γ-Lactams: Synthesis and Natural Sources Cite This: Org
Organic & Biomolecular Chemistry REVIEW Biologically active γ-lactams: synthesis and natural sources Cite this: Org. Biomol. Chem., 2016, 14, 10134 J. Caruano,a,b G. G. Mucciolib and R. Robiette*a Received 22nd June 2016, The γ-lactam moiety is present in a large number of natural and non-natural biologically active com- Accepted 5th October 2016 pounds. The range of biological activities covered by these compounds is very broad. Functionalized DOI: 10.1039/c6ob01349j γ-lactams are thus of high interest and have great potential in medicinal chemistry. This review provides a www.rsc.org/obc description of the title compounds by focusing on their synthesis, natural sources and biological activities. 1 Introduction antibiotics. To overcome this resistance issue it was necessary to move away from the β-lactam core. Knowing that a suitable The γ-lactam ring, also known as γ-butyrolactam, pyrrolidin-2- activated amide bond is essential for the activity (but not the one, azolidin-2-one or 2-oxopyrrolidine, is part of the core β-lactam ring), the attention turned naturally to γ-lactams and structure of a large number of natural and non-natural com- their analogues, and more specifically to bicyclic γ-lactams pounds covering a broad spectrum of biological activities. such as penem derivatives. Accordingly, γ-lactams are of primary interest in medicinal It is in 1986 that the first γ-lactam analogues of penicillins chemistry and many synthetic strategies have been disclosed active as antibiotics were reported, independently, by Baldwin – to access this structural moiety. et al. and researchers from Eli Lilly.1 4 According to Baldwin, The interest in this scaffold started with the observation of who developed compound A (Fig. -

Chemistry Explored
Issue 4 // Autumn 2015 bristol.ac.uk/chemistry THE GREAT LEAP FORWARD HOW SELF-REPAIRING TECHNOLOGY DEVELOPED BY BRISTOL CHEMISTS COULD CHANGE THE WORLD DIAL A MOLECULE: HOW SYNTHESISING A MOLECULE JUST GOT EASIER LIFE IN CHEMISTRY: TAKE A VIRTUAL TOUR OF THE SCHOOL Welcome Also in this issue… Welcome to the autumn issue of Chemistry Explored. This summer saw us say goodbye to students who graduated in July and headed off to pursue their goals after completing their undergraduate and postgraduate degrees with us. You can read about some of the careers that chemistry News leads to in this edition. 2015 Science Alive 03 As well as celebrating academic Thornbury Festival, latest from Tyntesfield 04 success, we’ve attracted widespread I’m a scientist, get me out of here! 05 media attention. Research carried out Summer in the labs, Fresh ideas for first years 06 into advanced composite materials leading to the development of self- Features healing technology had a huge amount Molecules made easy 07 of press coverage, which you can read Generation next 08 more about in this edition. Technology, heal thyself 10 We’ve also welcomed Professor Life in Chemistry 12 Jonathan Clayden who has recently Climbing for charity 14 joined us from Manchester. We’re Infographic 15 delighted that Jonathan has chosen Life through a lens 16 to move to Bristol and there will be a feature about him and his research University of Bristol Production Editor Steve O’Brien Art Editor School of Chemistry Elaine Knight-Roberts Editorial Director Dan group in the next edition. -
![Asymmetric Organocatalysis Stefan Jaroch,*[A] Hilmar Weinmann,*[A] and Kirsten Zeitler*[B]](https://docslib.b-cdn.net/cover/8130/asymmetric-organocatalysis-stefan-jaroch-a-hilmar-weinmann-a-and-kirsten-zeitler-b-4988130.webp)
Asymmetric Organocatalysis Stefan Jaroch,*[A] Hilmar Weinmann,*[A] and Kirsten Zeitler*[B]
DOI: 10.1002/cmdc.200700109 Asymmetric Organocatalysis Stefan Jaroch,*[a] Hilmar Weinmann,*[a] and Kirsten Zeitler*[b] Chemical synthesis is one of the key technologies underlying thesis and invaluable to pharmaceutical industry. Recent develop- modern drug discovery and development. For the design and ac- ments in the field of asymmetric catalysis point to a third class cessibility of novel structures and the rapid preparation of new of catalysts besides the established enzymes and metal com- test compounds and development candidates with often highly plexes, the so-called organocatalysts. In an effort to increase the complex chemical architecture, it is essential to use state-of-the- awareness within the community of medicinal and process chem- art chemical synthesis technologies. As the number of chiral ists as well as to learn more about recent developments in this drugs is increasing, asymmetric synthesis and efficient chiral sep- rapidly evolving field, the Schering Foundation organized a sym- aration technologies are steadily gaining importance. Regarding posium on “Organocatalysis”, which took place in Berlin on April asymmetric catalysis, enzymatic transformations, and reactions 18–20, 2007. This minireview summarizes some of the important employing metal, catalysts are important tools for organic syn- results presented at the symposium. Introduction Chemical synthesis is one of the key technologies underlying modern drug discovery and development. For the design and accessibility of novel structures and the rapid preparation of new test compounds and development candidates with often highly complex chemical architecture, it is essential to use state-of-the-art chemical synthesis technologies. As the Scheme 1. Asymmetric Robinson annulation catalyzed by l-proline leading [1] [2] number of chiral drugs is increasing, asymmetric synthesis to the steroid CD fragment. -

2013 NOS Book COLOR Final
43 rd National Organic Chemistry Symposium Statue of George Washington outside Meany Hall looking west to the Olympic Mountains. Photo courtesy of Loyd C. Heath. University of Washington Seattle, Washington June 23-27, 2013 For information on the Division and other items of interest, visit our website: http://organicdivision.org Graduate Research Symposium (GRS) The GRS provides an opportunity for 50-75 graduate students in organic chemistry to interact with leaders from academia, industry, various funding agencies, and publishers at a single venue will take place this year at the University of Delaware on July 25–28. Watch the monthly DOC newsletters for the call for applications for 2014. Technical Achievement in Organic Chemistry (TAOC) Awards Nominations for the TAOC Awards to recognize outstanding contributions to the field of organic chemistry from accomplished Junior-level chemists (i.e., those who do not have a Ph.D. degree or equivalent). Awards are given at the ACS Meeting. Nominations typically open late fall and are accepted through the online nomination form until mid January. Travel Awards Travel awards ($600 each) for graduate students, undergraduates, and faculty at PUIs for travel to ACS National Meetings to present their work within the Organic Division's Symposia/Poster Sessions. Application deadlines for National Meetings will typically be November 15th for the Spring meeting and May 1st for the Fall Meeting. Videos: Eminent Organic Chemists, The Human Side Chemical historian Jeffrey I. Seeman has conducted a series of video interviews with prominent organic chemists (to date, 15 have been released). One goal of these interviews was to reveal the human side of these scientists by exploring their values, and breadth of experiences. -

Catalytic, Asymmetric Sulfur Ylide-Mediated Epoxidation of Carbonyl Compounds: Scope, Selectivity, and Applications in Synthesis VARINDER K
Acc. Chem. Res. 2004, 37, 611-620 Catalytic, Asymmetric Sulfur Ylide-Mediated Epoxidation of Carbonyl Compounds: Scope, Selectivity, and Applications in Synthesis VARINDER K. AGGARWAL* AND FIGURE 1. CAROLINE L. WINN While there are many examples of the two-step process School of Chemistry, Cantock's Close, University of Bristol, involving a Wittig reaction followed by asymmetric oxida- Bristol BS8 1TS, U.K. tion, the one-step sulfur ylide route had not been widely Received December 8, 2003 used. We felt that if we could render the process both catalytic and asymmetric, the advantages of the new one- step route could begin to compete with the more tradi- ABSTRACT The reaction of sulfur ylides with carbonyl compounds to give tional oxidative approach. epoxides is an important synthetic method. This Account charts the recent advances in rendering this process both asymmetric and catalytic. Two catalytic methods have been developed: the first Introduction to Sulfur Ylide-Mediated involving the reaction of a sulfide with an alkyl halide in the Epoxidation presence of a base and aldehyde and the second involving the Johnson reported the first literature example of a sulfur reaction of a sulfide with a diazo compound or diazo precursor in 6 the presence of a metal catalyst and aldehyde. These catalytic ylide-mediated epoxidation reaction in 1958. Reaction of methods coupled with suitable chiral sulfides provide a new the stable and isolable 9-dimethylsulfonium fluorenylide catalytic asymmetric epoxidation process for the preparation of 1 (Figure 2) with 4-nitrobenzaldehyde did not afford an epoxides. The scope of the two catalytic processes is discussed alkene, which had been expected on the basis of the together with the factors that influence both relative and absolute stereochemistry. -

50 Years of Zweifel Olefination: a Transition-Metal-Free Coupling
SYNTHESIS0039-78811437-210X Georg Thieme Verlag Stuttgart · New York 2017, 49, 3323–3336 short review 3323 en Syn thesis R. J. Armstrong, V. K. Aggarwal Short Review 50 Years of Zweifel Olefination: A Transition-Metal-Free Coupling Roly J. Armstrong 0000-0023-7590-61X Varinder K. Aggarwal* 0000-0030-3446-430 School of Chemistry, University of Bristol, Cantock’s Close, Bristol, BS8 1TS, UK [email protected] Dedicated to Professor Herbert Mayr on the occasion of his 70th birthday Received: 15.05.2017 Accepted after revision: 16.05.2017 Published online: 11.07.2017 DOI: 10.1055/s-0036-1589046; Art ID: ss-2017-z0328-sr License terms: Abstract The Zweifel olefination is a powerful method for the stereo- selective synthesis of alkenes. The reaction proceeds in the absence of a transition-metal catalyst, instead taking place by iodination of vinyl bo- ronate complexes. Pioneering studies into this reaction were reported in 1967 and this short review summarizes developments in the field over the past 50 years. An account of how the Zweifel olefination was modified to enable the coupling of robust and air-stable boronic esters Varinder K. Aggarwal (right) studied chemistry at Cambridge Univer- is presented followed by a summary of current state of the art develop- sity and received his Ph.D. in 1986 under the guidance of Dr Stuart ments in the field, including stereodivergent olefination and alkynyla- Warren. After postdoctoral studies (1986–1988) under Professor Gil- tion. Finally, selected applications of the Zweifel olefination in target- bert Stork, Columbia University, he returned to the UK as a Lecturer at oriented synthesis are reviewed. -

The Catalytic Mechanism of a Natural Diels-Alderase Revealed in Molecular Detail. Journal of the American Chemical Society 2016, 138(19), 6095-6098
Byrne MJ, Lees NR, Han LC, van der Kamp MW, Mulholland AJ, Stach JEM, Willis CL, Race PR. The Catalytic Mechanism of a Natural Diels-Alderase Revealed in Molecular Detail. Journal of the American Chemical Society 2016, 138(19), 6095-6098 Copyright: This document is the Accepted Manuscript version of a Published Work that appeared in final form in Journal of the American Chemical Society, copyright © American Chemical Society after peer review and technical editing by the publisher. To access the final edited and published work see http://dx.doi.org/ 10.1021/jacs.6b00232 DOI link to article: http://dx.doi.org/10.1021/jacs.6b00232 Date deposited: 21/09/2016 Embargo release date: 03 May 2017 Newcastle University ePrints - eprint.ncl.ac.uk The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail Matthew J. Byrne,†,‡ Nicholas R. Lees,§ Li-Chen Han,‡,§ Marc W. van der Kamp,†,‡,§ Adrian J. Mulholland,‡,§ James E.M. Stach,, Christine L. Willis,‡,§ and Paul R. Race*,†,‡ †School of Biochemistry, Biomedical Sciences Building, University Walk, University of Bristol, BS8 1TD, UK. ‡BrisSynBio Synthetic Biology Research Centre, Life Sciences Building, Tyndall Avenue, University of Bristol, BS8 1TQ, UK. §School of Chemistry, Cantock’s Close, University of Bristol, BS8 1TS, UK. School of Biology, Ridley Building, Newcastle University, NE1 7RU, UK. Centre for Synthetic Biology and the Bioeconomy, Baddiley-Clark Building, Newcastle University, NE2 4AX, UK. Supporting Information Placeholder ABSTRACT: The Diels-Alder reaction, a [4+2] have evolved Diels-Alderase activity, and in particular, cycloaddition of a conjugated diene to a dienophile, is how such catalysis is achieved at the molecular level, is one of the most powerful reactions in synthetic crucial for the development of efficient protein Diels- 15-18,21 chemistry.