Chalcogenides As Organocatalysts Eoghan M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Treatment Processes for Hydrogen Sulfide
Removing the Stink: Advanced Treatment Processes for Hydrogen Sulfide Clayton Johnson, Christine Owen, Luke Mulford, Shahnawaz Sinha, Zaid Chowdhury, Andre Dieffenthaller, and Andrew Coleman ampa Bay Water supplies drinking The final alternative under considera- water to more than 2 million people in tion is biological oxidation followed by chlo- Clayton Johnson is a project engineer in Tthe greater Tampa Bay and adjacent rination and ultrafiltration following biolog- the Tampa office of the environmental areas. Approximately 60 percent of its source ical oxidation prior to distribution. engineering firm Malcolm Pirnie Inc. water comes from groundwater supplies. This article will discuss preliminary Christine Owen is a water quality assur- ance officer with Tampa Bay Water. Luke Groundwater in some portions of the region findings of this ongoing pilot study, including Mulford is a water quality engineer with has a moderate amount (about 2 mg/L as operational variables and effectiveness of the Hillsborough County Water Resource total sulfides) of hydrogen sulfide. Tampa Bay proposed treatment processes for hydrogen Services. Shahnawaz Sinha is a project Water currently provides water to a water sulfide removal. As many Florida utilities are engineer with Malcolm Pirnie in Phoenix, treatment facility that utilizes aeration fol- faced with the challenge of removing hydro- Arizona. Zaid Chowdhury is a senior lowed by biological oxidation to remove gen sulfide from their groundwater, prelimi- associate with Malcolm Pirnie in Phoenix. hydrogen sulfide. nary results of this study will be broadly Andre Dieffenthaller is a senior associate This combined practice (Figure 1) is applicable. Results from this study will pro- with Malcolm Pirnie in Schaumburg, effective, but there are occasional reductions in vide useful information to water utilities that Illinois. -

Platinum-Group Elements and Gold in Sulfide Melts from Modern Arc Basalt (Tolbachik Volcano, Kamchatka)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by The Australian National University ÔØ ÅÒÙ×Ö ÔØ Platinum-group elements and gold in sulfide melts from modern arc basalt (Tolbachik volcano, Kamchatka) M. Zelenski, V.S. Kamenetsky, J.A. Mavrogenes, L.V. Danyushevsky, D. Matveev, A.A. Gurenko PII: S0024-4937(17)30290-6 DOI: doi:10.1016/j.lithos.2017.08.012 Reference: LITHOS 4395 To appear in: LITHOS Received date: 30 May 2017 Accepted date: 21 August 2017 Please cite this article as: Zelenski, M., Kamenetsky, V.S., Mavrogenes, J.A., Danyu- shevsky, L.V., Matveev, D., Gurenko, A.A., Platinum-group elements and gold in sul- fide melts from modern arc basalt (Tolbachik volcano, Kamchatka), LITHOS (2017), doi:10.1016/j.lithos.2017.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Platinum-group elements and gold in sulfide melts from modern arc basalt (Tolbachik volcano, Kamchatka) M. Zelenski a, V.S. Kamenetsky a,b,*, J.A. Mavrogenes c, L.V. Danyushevsky b, D. Matveev d, A.A. Gurenko e a Institute of Experimental Mineralogy RAS, Chernogolovka 142432, Russia b Earth Sciences and CODES, University of Tasmania, Private Bag 79, Hobart, TAS 7001, Australia c Research School of Earth Sciences, Australian National University, Canberra, ACT 2601, Australia d Institute of Solid State Physics RAS, Chernogolovka 142432, Russia e Centre de Recherches Pétrographiques et Géochimiques (CRPG), UMR 7358, Université de Lorraine, 54501 Vandoeuvre-lès-Nancy, France * Corresponding author. -

The Determination of Sulfate and Sulfide Sulfur in Rocks Or Minerals
The Determination of Sulfate and Sulfide Sulfur in Rocks or Minerals By ANGELINA C. VLISIDIS CONTRIBUTIONS TO GEOCHEMISTRY GEOLOGICAL SURVEY BULLETIN 1214-D UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1966 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 15 cents (paper cover) CONTENTS Page Abstract_____--__-___-_______-__---____,__-_-__-_---_-_______-_- Dl Introduction. ______________________________________________________ 1 Preparations. _________._.-.__-_-.__.._-_---__----.________._.._____ 2 Standard samples____________________________________________ 2 Reagents. _______________.-_-___-____-__-_-__-_-___-_______-_- 2 Procedure._______________________________________________________ 2 Results__ __________-______-_____----__--_--_----_-_-_-___-___--_ 3 References.._ _____________________________________________________ 5 TABLE Page TABLE 1. Results of sulfide and sulfate sulfur analyses in which varying amounts of a sulfate standard were added to sulfide minerals.. _ D4 m 209-517 66 CONTRIBUTIONS TO GEOCHEMISTRY THE DETERMINATION OF SULFATE AND SULFIDE SULFUR IN ROCKS OR MINERALS By ANGELINA C. VLISEDIS , ABSTRACT A method for the determination of sulfate and sulfide sulfur that occur together in rocks or minerals is presented. All the sulfate sulfur is converted to barium sulfate in an inert atmosphere to prevent oxidation of any sulfide sulfur. Cadmium chloride is added to precipitate any sulfide ion that may be liberated. The sulfate sulfur is then measured indirectly by the determination of the barium and is therefore unaffected by any. subsequent oxidation of the sulfide sulfur. -

Common Name: SELENIUM SULFIDE HAZARD SUMMARY
Common Name: SELENIUM SULFIDE CAS Number: 7446-34-6 RTK Substance number: 1653 DOT Number: UN 2657 Date: October 1995 Revision: October 2001 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Selenium Sulfide can affect you when breathed in and by * If you think you are experiencing any work-related health passing through your skin. problems, see a doctor trained to recognize occupational * Selenium Sulfide should be handled as a CARCINOGEN- diseases. Take this Fact Sheet with you. -WITH EXTREME CAUTION. * Contact can irritate the eyes with possible eye damage. WORKPLACE EXPOSURE LIMITS * Breathing Selenium Sulfide can irritate the nose and The following exposure limits are for Selenium compounds throat. (measured as Selenium): * High exposure may cause headache, nausea, vomiting, garlic odor of the breath, metallic taste and coated tongue. OSHA: The legal airborne permissible exposure limit * Repeated exposure can cause pallor, nervousness and (PEL) is 0.2 mg/m3 averaged over an 8-hour mood changes. workshift. * Selenium Sulfide may damage the liver and kidneys. NIOSH: The recommended airborne exposure limit is IDENTIFICATION 0.2 mg/m3 averaged over a 10-hour workshift. Selenium Sulfide is a bright orange powder. It is used in medicated shampoos. ACGIH: The recommended airborne exposure limit is 3 0.2 mg/m averaged over an 8-hour workshift. REASON FOR CITATION * Selenium Sulfide is on the Hazardous Substance List * Selenium Sulfide may be a CARCINOGEN in humans. because it is regulated by OSHA and cited by ACGIH, There may be no safe level of exposure to a carcinogen, so DOT, NIOSH, NTP, DEP, HHAG and EPA. all contact should be reduced to the lowest possible level. -

Hydrogen Sulfide Public Health Statement
PUBLIC HEALTH STATEMENT Hydrogen Sulfide Division of Toxicology and Human Health Sciences December 2016 This Public Health Statement summarizes what is known about hydrogen sulfide such as possible health effects from exposure and what you can do to limit exposure. The U.S. Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are sites targeted for long-term federal clean-up activities. U.S. EPA has found hydrogen sulfide in at least 34 of the 1,832 current or former NPL sites. The total number of NPL sites evaluated for hydrogen sulfide is not known. But the possibility remains that as more sites are evaluated, the sites at which hydrogen sulfide is found may increase. This information is important because these future sites may be sources of exposure, and exposure to hydrogen sulfide may be harmful. If you are exposed to hydrogen sulfide, many factors determine whether you’ll be harmed. These include how much you are exposed to (dose), how long you are exposed (duration), and how you are exposed (route of exposure). You must also consider the other chemicals you are exposed to and your age, sex, diet, family traits, lifestyle, and state of health. WHAT IS HYDROGEN SULFIDE? Hydrogen sulfide (H2S) is a flammable, colorless gas that smells like rotten eggs. People usually can smell hydrogen sulfide at low concentrations in air, ranging from 0.0005 to 0.3 parts hydrogen sulfide per million parts of air (ppm). At high concentrations, a person might lose their ability to smell it. -

Kinetics of the Ozonation of Dimethyl Sulfide in the Gas Phase
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1973 Kinetics of the ozonation of dimethyl sulfide in the gas phase Robert John Moody The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Moody, Robert John, "Kinetics of the ozonation of dimethyl sulfide in the gas phase" (1973). Graduate Student Theses, Dissertations, & Professional Papers. 8125. https://scholarworks.umt.edu/etd/8125 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. KINETICS OF THE OZONATION OF DIMETHYL SOLFIDB IN THE GAS PHASE by Robert J, Moody B.S., University of Montana, 1968 Presented in partial fulfillment of the requirements for the degree of Master of Science UNIVERSITY OF MONTANA 1973 Approved by: Chairman, Board of Examiners Deanf 'Graduait Schoo Date 7 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: EP38926 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. -

Hydrogen Sulfide Fact Sheet
Hydrogen Sulfide Fact Sheet What is hydrogen sulfide? Hydrogen sulfide (H 2S) occurs naturally in crude petroleum, natural gas, volcanic gases, and hot springs. It can also result from bacterial breakdown of organic matter. It is also produced by human and animal wastes. Bacteria found in your mouth and gastrointestinal tract produce hydrogen sulfide from bacteria decomposing materials that contain vegetable or animal proteins. Hydrogen sulfide can also result from industrial activities, such as food processing, coke ovens, kraft paper mills, tanneries, and petroleum refineries. Hydrogen sulfide is a flammable, colorless gas with a characteristic odor of rotten eggs. It is commonly known as hydrosulfuric acid, sewer gas, and stink damp. People can smell it at low levels. What happens to hydrogen sulfide when it enters the environment? • Hydrogen sulfide is released primarily as a gas and spreads in the air. • Hydrogen sulfide remains in the atmosphere for about 18 hours. • When released as a gas, it will change into sulfur dioxide and sulfuric acid. • In some instances, it may be released as a liquid waste from an industrial facility. How might I be exposed to hydrogen sulfide? • You may be exposed to hydrogen sulfide from breathing contaminated air or drinking contaminated water. • Individuals living near a wastewater treatment plant, a gas and oil drilling operation, a farm with manure storage or livestock confinement facilities, or a landfill may be exposed to higher levels of hydrogen sulfide. • You can be exposed at work if you work in the rayon textiles, petroleum and natural gas drilling and refining, or wastewater treatment industries. -

Oxidation of Sulfide Minerals. V. Galena, Sphalerite and Chalcogite
Canadian Mineralogist Vol. 18,pp. 365-372(1980) OXIDATIONOF SULFIDEMINERALS. V. GALENA,SPHALERITE AND CHALCOGITE H.F. STEGBR eup L.E. DESJARDINS Mineral SciencesLaboratories, Canada Centre lor Mineral and Energy Technology, Department ol Energy, Mines and Resources,Ottawa, Ontaio KIA OGI AssrRecr long-term stability of sulfide-bearing ores and concentrates.Part of this study was concerned Samples of galena, sphalerite and cbalcocite were with the nature of the products and kinetics of oxidized at 52oC and, 68Vo of relative humidity the oxidation of the commonly encountered periods to five weeks, and the prqd- in air for up sulfide minerals. The oxidation of pyrite, chal- for metal and sulfur-bearing ucts were analyzed pyrrhotite at 52oC and, 687o of. species. Galena is. oxidized to PbSOa, sphalerite copyrite and (RH) has already been in- to ZnSO. * FezO, if iron-bearing, and chalcocite relative humidity to CuO and CuS. The oxidation of galena and vestigated (Steger & Desjardins 1978). This re- sphalerite proceeds according to a linear rate potr summarizes the results of the study 9f tle law: that of chalcocite leads to the formation of a bxidation of galena, sphalerite and chalcocite coherent product layer impenetrable to Oz and HrO under the same conditions. vapor. The air oxidation of galena at relatively low without Keywords: air oxidation, oxidation products, sul- temperatures has been investigated fide minerals, galena, sphalerite, chalcocite. reaching a consensuson the nature of the oxida- tion product. Hagihara (1952), using a re- Sourvrlrnp flection electron-diffraction technique, and Kirkwood & Nutting (1965), using a trans- Nous avons 6tudi6 I'oxydation dans I'air d'6chan- mission electron-diffraction technique, found tillons de galdne, de sphal6rite et de chalcocite i this product to be PbSOo,whereas Leia et al. -

Fact Sheet: Hydrogen Sulfide from Landfills
Fact Sheet: Hydrogen Sulfide from Landfills How might I be exposed to hydrogen sulfide from a landfill? Hydrogen sulfide is a flammable, colorless gas with a characteristic odor of rotten eggs. It is heavier than air and is commonly known as hydrosulfuric acid, sewer gas, and stink damp. Hydrogen sulfide occurs both naturally and from industrial processes. Hydrogen sulfide can be released from landfills and people living near landfills may be exposed to it by breathing it in. How can hydrogen sulfide affect my health? Exposure to low concentrations within the range of 30 parts of hydrogen sulfide in one billion parts of air (ppb) may cause irritation to the eyes, nose, or throat. It may also cause difficulty breathing for some individuals with respiratory problems, such as asthmatics. DOH uses the Minimal Risk Levels (MRLs) developed by the federal Agency for Toxic Substances and Disease Registry (ATSDR) to assess the possibility of non-cancer health effects. An MRL is an estimate of the daily human exposure to a hazardous substance, at or below which, that substance is unlikely to pose a measurable risk of adverse, non-cancer health effects. These particular values have many safety factors built in and the actual levels where health effects would be observed are much higher. The acute MRL, which represents short time periods of exposure (less than 15 days), is 70 ppb. Per the study that established this acute MRL, it should be noted that the actual level where health effects (respiratory problems and headaches) were observed was 2,000 ppb. The intermediate MRL is defined for exposure periods of 15 to 365 days. -

Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001
CONFERENCE REPORT 732 CHIMIA 2001, 55, No.9 CONFERENCE REPORT Chimia 55 (2001) 732-736 © Schweizerische Chemische Gesellschaft ISSN 0009-4293 Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001 Christian G. Bochet* Keywords: CERC3 . Champery . European chemistry' Organic synthesis· Workshop The chairmen of the European Re- from the chiral centers, had a strong im- The day was concluded with the first search Councils' Chemistry Committees pact on the geometry of the dimeric cata- plenary lecture, by Professor Varinder (CERC3) elected organic synthesis as lyst; these effects could be so strong that Aggarwal (University of Bristol, UK). this year's topic for their annual work- similar catalysts varying only by one sub- He explained the asymmetric epoxida- shop, and Switzerland as the host coun- stituent gave opposite enantiomers [1]. tion reaction, in which, instead of using try. The conference chairman, Prof. The afternoon session was concluded the classical strategy (i.e. oxidation of an Philippe Renaud made the excellent by Petri Pihko (The Scripps Research In- alkene), the key step is a carbon-carbon choice of Champery for hosting the stitute, La Jolla, USA), on the synthesis bond forming process (the so-called Co- workshop; the combination of his enthu- of Azaspiracid (Fig. 1), a very potent tox- rey-Chaykovsky epoxidation). The beau- siasm, efficient organization and the in, isolated from Irish mussels. The FGHI ty of this reaction, very simple in its ex- beautiful alpine scenery made this meet- spirocyclic portion proved to be a very perimental realization, is revealed by the ing a really unique event. -
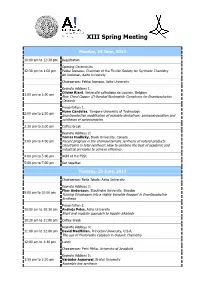
XIII Spring Meeting
XIII Spring Meeting Monday, 24 June, 2013 10:00 am to 12:30 pm Registration Opening Ceremonies 12:30 pm to 1:00 pm Pekka Joensuu, Chairman of the Finnish Society for Synthetic Chemistry Ari Koskinen, Aalto University Chairperson: Pekka Joensuu, Aalto University Keynote Address 1: Olivier Riant, Université catholique de Louvain, Belgium 1:00 pm to 2:00 pm New Chiral Copper (I)-Bonded Nucleophile Complexes for Enantioselective Catalysis Presentation 1: Nuno Candeias, Tampere University of Technology 2:00 pm to 2:30 pm Enantioselective modification of oxindole derivatives: aminooxygenation and syntheses of spirooxindoles 2:30 pm to 3:00 pm Coffee break Keynote Address 2: Tomas Hudlicky, Brock University, Canada 3:00 pm to 4:00 pm Recent progress in the chemoenzymatic synthesis of natural products. Constraints in total synthesis: How to combine the best of academic and industrial principles to achieve efficiency. 4:00 pm to 5:00 pm AGM of the FSSC 5:00 pm to 7:00 pm Get together Tuesday, 25 June, 2013 Chairperson: Reija Jokela, Aalto University Keynote Address 3: Pher Andersson, Stockholm University, Sweden 9:00 am to 10:00 am Turning Dihydrogen into a Highly Versatile Reagent in Enantioselective Synthesis Presentation 2: 10:00 am to 10:30 am Andrejs Pelss, Aalto University Short and modular approach to lepadin alkaloids 10:30 am to 11:00 am Coffee Break Keynote Address 4: 11:00 am to 12:00 am David MacMillan, Princeton University, U.S.A. The use of Photoredox Catalysis in Organic Chemistry 12:00 am to 1:30 pm Lunch Chairperson: Petri Pihko, -

FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr
FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr. Lee D. Arnold 1987, Ph.D. Serine β-Lactones in Syntheses of Amino Acids None President & CEO of Discovery Elucidations [email protected] Dr. Hanaa Assil 1989, M.Sc. Solid Supports for Azodicarboxylates in Mitsunobu Reactions n/a Unknown Unknown and Synthesis of Amino Acids Dr. Karine Auclair 1999, Ph.D. Biosynthetic Studies on the Polyketide Lovastatin: Enzyme- Prof. P. Ortiz De Montellano, Department Assoc. Professor, Department of Chemistry, McGill University, [email protected] Catalyzed Diels-Alder Reactions of Pharmaceutical Chemistry, University of Montreal, Que., Canada California at San Francisco, USA Dr. Jennifer Blunston (nee Caplan) 2001, Ph.D. Diaminopimelic Acid Analogues as Inhibitors of Enzymes Prof. D. Waisman, Departments of Stay-at-home mom, raising son Rhys. Previously Jennifer [email protected] Involved in Bacterial Lysine Biosynthesis Biochemistry and Oncology, University of Blunston was Assistant Professor of Chemistry, St. Mary's Calgary, Canada University College, Calgary, AB, Canada Dr. Marc Boudreau 2007, Ph.D. Nucleoside Dicarboxylates as Mimics of Diphosphates and Prof. Hagan Bayley, Oxford University Postdoc with Professor Shahriar Mobashery, University of Notre [email protected] Disulfide Bond Replacement in Pediocin PA1 Dame Dr. Doug Burr 2006, Ph.D. Studies of the in vitro Activity of Lovastatin Nonaketide Prof. David Sherman, Dept. Medicinal Research scientist at Perkin Elmer, Boston, Massachusetts [email protected] Synthase Chemistry, University of Michigan, Ann Arbor, MI Dr. Hengmiao (Henry) Cheng 1992, Ph.D. Mechanism and Inhibition of Peptidylglycine α-Hydroxlating Prof. E.J. Corey, Department of Chemistry, Senior Research Investigator, Pfizer Global Research and [email protected] Monooxygenase Harvard University Development, Pfizer Inc., Groton, CT, USA Dr.