FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Masthead 2019
Masthead AngewandteA Journal of the German Chemical Society International Edition Chemie Editorial Board Chair: Annette G. Beck-Sickinger, Universität Leipzig Michael Brands, Bayer (Berlin) Editor: Neville A. Compton Holger Braunschweig, Julius-Maximilians-Universität (Würzburg) Martin Brudermüller, BASF (Ludwigshafen) Deputy Editors: Frank Maaß, Nathalie Weickgenannt Thomas Carell, Ludwig-Maximilians-Universität München Klaus Griesar, Merck (Darmstadt) Editorial Office: Senior Associate Editors: Jens Ackermann, Stefan Grimme, Universität Bonn Jonathan Faiz, Tamaryin Godinho, Hansjörg Grützmacher, Eidgenöss. Techn. Hochschule Zürich Nicole Harrington-Frost, Stephen Horner, (Switzerland) Volker Jacob, Guy Richardson, Rainer Haag, Freie Universität Berlin Rachel Schmidt-Radde, Diane Smith, Christian W. Kohlpaintner, Clariant (Pratteln, Switzerland) Xin Su, Suzanne Tobey Walter Leitner, Rheinisch-Westfälische Technische Hochschule Aachen Senior Web Editor: Mario Müller Wolfgang Parak, Universität Marburg Erwin Reisner, University of Cambridge (UK) Associate Editors: Eric Castro, Wolfgang Schnick, Ludwig-Maximilians-Universität München Arno Knappschneider, Kim Meyer Ferdi Schüth, Max-Planck-Institut für Kohlenforschung (Mülheim) Senior Assistant Editors: Gary Battle, Wolfgang Schuhmann, Ruhr-Universität Bochum Christiane Walter Harald Schwalbe, Johann Wolfgang Goethe-Universität Frankfurt Assistant Editors: Lisa Pecher, Petra Schwille, Max-Planck-Institut für Biochemie (Martinsried) Polina Smirnov, Laura Woodward Armido Studer, Westfälische -

Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001
CONFERENCE REPORT 732 CHIMIA 2001, 55, No.9 CONFERENCE REPORT Chimia 55 (2001) 732-736 © Schweizerische Chemische Gesellschaft ISSN 0009-4293 Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001 Christian G. Bochet* Keywords: CERC3 . Champery . European chemistry' Organic synthesis· Workshop The chairmen of the European Re- from the chiral centers, had a strong im- The day was concluded with the first search Councils' Chemistry Committees pact on the geometry of the dimeric cata- plenary lecture, by Professor Varinder (CERC3) elected organic synthesis as lyst; these effects could be so strong that Aggarwal (University of Bristol, UK). this year's topic for their annual work- similar catalysts varying only by one sub- He explained the asymmetric epoxida- shop, and Switzerland as the host coun- stituent gave opposite enantiomers [1]. tion reaction, in which, instead of using try. The conference chairman, Prof. The afternoon session was concluded the classical strategy (i.e. oxidation of an Philippe Renaud made the excellent by Petri Pihko (The Scripps Research In- alkene), the key step is a carbon-carbon choice of Champery for hosting the stitute, La Jolla, USA), on the synthesis bond forming process (the so-called Co- workshop; the combination of his enthu- of Azaspiracid (Fig. 1), a very potent tox- rey-Chaykovsky epoxidation). The beau- siasm, efficient organization and the in, isolated from Irish mussels. The FGHI ty of this reaction, very simple in its ex- beautiful alpine scenery made this meet- spirocyclic portion proved to be a very perimental realization, is revealed by the ing a really unique event. -
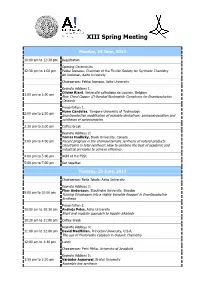
XIII Spring Meeting
XIII Spring Meeting Monday, 24 June, 2013 10:00 am to 12:30 pm Registration Opening Ceremonies 12:30 pm to 1:00 pm Pekka Joensuu, Chairman of the Finnish Society for Synthetic Chemistry Ari Koskinen, Aalto University Chairperson: Pekka Joensuu, Aalto University Keynote Address 1: Olivier Riant, Université catholique de Louvain, Belgium 1:00 pm to 2:00 pm New Chiral Copper (I)-Bonded Nucleophile Complexes for Enantioselective Catalysis Presentation 1: Nuno Candeias, Tampere University of Technology 2:00 pm to 2:30 pm Enantioselective modification of oxindole derivatives: aminooxygenation and syntheses of spirooxindoles 2:30 pm to 3:00 pm Coffee break Keynote Address 2: Tomas Hudlicky, Brock University, Canada 3:00 pm to 4:00 pm Recent progress in the chemoenzymatic synthesis of natural products. Constraints in total synthesis: How to combine the best of academic and industrial principles to achieve efficiency. 4:00 pm to 5:00 pm AGM of the FSSC 5:00 pm to 7:00 pm Get together Tuesday, 25 June, 2013 Chairperson: Reija Jokela, Aalto University Keynote Address 3: Pher Andersson, Stockholm University, Sweden 9:00 am to 10:00 am Turning Dihydrogen into a Highly Versatile Reagent in Enantioselective Synthesis Presentation 2: 10:00 am to 10:30 am Andrejs Pelss, Aalto University Short and modular approach to lepadin alkaloids 10:30 am to 11:00 am Coffee Break Keynote Address 4: 11:00 am to 12:00 am David MacMillan, Princeton University, U.S.A. The use of Photoredox Catalysis in Organic Chemistry 12:00 am to 1:30 pm Lunch Chairperson: Petri Pihko, -

Bios and Abstracts of RSC Speakers
Colin Bain graduated in Natural Sciences from Cambridge before crossing the Atlantic to undertake his PhD at Harvard with Prof. George Whitesides. He then returned to Cambridge as a Royal Society Fellow, working with Dr. Paul Davies to set up the UK’s first sum-frequency spectrometer. In 1991 he moved to Oxford as a University Lecturer and Fellow of Magdalen College. In 2005 he took up his current position of Professor of Chemistry at Durham University. His research interests lie principally in the chemistry and physics of interfaces, with applications in detergents, personal care products, printing, spraying and lubrication. Prof. Bain has received the Harrison, Corday-Morgan and Tilden Prizes from the Royal Society of Chemistry as well as awards in Japan and Australia. In 2005 he was honoured to deliver the annual McBain Lecture at the National Chemical Laboratory in Pune. He has collaborated with the Indian Institute of Science in Bangalore for a number of years and currently holds a joint UKIERI award with the IISc. Professor Colin Bain Since 2008, he has been a Director of the Institute of Advanced Study at Department of Chemistry Durham – an institute that seeks to promote interdisciplinary dialogue Durham University and research collaborations across the humanities, sciences and social South Road sciences. He has been actively involved in many aspects of industry- Durham DH1 3LE academia interactions, through research collaborations with industry, as U.K. a Director of the Oxford Science Park and as a scientific advisor to a [email protected] Phone (+44) 191 334 2138 venture capital partnership. -

Bob Eisenberg (More Formally, Robert S
Bob Eisenberg (more formally, Robert S. Eisenberg) Curriculum Vitae September 11, 2021 Maintained with loving care by John Tang, all these years, with thanks from Bob! Address 7320 Lake Street Unit 5 River Forest IL 60305 USA or PO Box 5409 River Forest IL 60305 USA or Department of Physiology & Biophysics Rush University 1750 West Harrison, Room 1519a Jelke Chicago IL 60612 or Dept of Applied Mathematics Room 106D Pritzker Center, corner of State and 31st Street, Illinois Institute of Technology, Chicago IL 60616 Phone numbers Voice: +1 (708) 932 2597; Rush Department: Voice +1 (312)-942-6454; Rush FAX: (312)-942-8711 FAX to email: (801)-504-8665 Skype name: beisenbe Email: [email protected] Other email:[email protected], [email protected], [email protected] Scopus ID’s are 55552198800 and 7102490928. NIH COMMONS name is BEISENBE. ORCID identifier is 0000-0002-4860-5434 Web of Science ResearcherID is: G-8716-2018 and/or P-6070-2019 Publons Public Profile G-8716-2018 Robert S. Eisenberg https://publons.com/researcher/1941224/robert-s-eisenberg/ NIH maintained “My Bibliography: Bob Eisenberg” at http://goo.gl/Z7a2V7 or https://www.ncbi.nlm.nih.gov/myncbi/browse/collection/47999805/?sort=date&direction=ascending Education Elementary School: New Rochelle, New York High School, 1956-59. Horace Mann School, Riverdale, New York City, graduated in three years with honors and awards in Biology, Chemistry, Physics, Mathematics, Latin, English, and History. An interviewer of J.R. Pappenheimer, Professor of Physiology, Harvard Medical School, on American Heart Sponsored television program, ~1957. p. 1 RS Eisenberg September 11, 2021 Undergraduate, 1959-62. -

Job Description and Person Specificationselection Criteria
_________________________________________________________________________ DEPARTMENT OF CHEMISTRY Job description and selection criteria Job title Postdoctoral Research Assistant Division Mathematics, Physical and Life Sciences Department Department of Chemistry Department of Chemistry, Chemical Biology, South Parks Road, Location Oxford, OX1 Grade and salary Grade 7: £29,541 - £32,267 per annum Hours Full time Contract type Fixed-term (there is funding for this post for 1 year) Reporting to Professor Hagan Bayley http://research.chem.ox.ac.uk/hagan-bayley.aspx Vacancy reference 106353 Additional The post is available immediately information Introduction The University The University of Oxford is a complex and stimulating organisation, which enjoys an international reputation as a world-class centre of excellence in research and teaching. It employs over 10,000 staff and has a student population of over 21,000. Most staff are directly appointed and managed by one of the University’s 130 departments or other units within a highly devolved operational structure - this includes 5,900 ‘academic- related’ staff (postgraduate research, computing, senior library, and administrative staff) and 2,820 ‘support’ staff (including clerical, library, technical, and manual staff). There are also over 1,600 academic staff (professors, readers, lecturers), whose appointments are in the main overseen by a combination of broader divisional and local faculty board/departmental structures. Academics are generally all also employed by one of the 38 constituent colleges of the University as well as by the central University itself. Our annual income in 2010/11 was £919.6m. Oxford is one of Europe's most innovative and entrepreneurial universities: income from external research contracts exceeds £376m p.a., and more than 70 spin-off companies have been created. -

Abstracts for ICP2019
The 29th International Conference on Photochemistry Boulder, Colorado • July 21 – 26, 2019 ABSTRACTS The 29th International Conference on Photochemistry Boulder, Colorado July 21 – 26, 2019 PLENARY SPEAKER ABSTRACTS PLENARY PRESENTATIONS ICP2019 Code: Plenary Presenter: Hiroshi MASUHARA (TWN) Abstract Title: Optical Manipulation in Chemistry Co-Author(s): Abstract: Photochemistry and spectroscopy study various dynamics and mechanism of molecules and materials induced by their interaction with light. Optical force is another interaction between light and matter, which was experimentally confirmed by Lebedev in late 19th century. Utilizing this force Ashkin proposed as “Optical Tweezers” in 1986 and demonstrated high potential in application to bio-science, which was awarded Nobel Prize Physics last year. Optical force was also expected to control molecular motion in solution and eventually chemical reaction. In 1988 we started to explore new molecular phenomena characteristic of optical force by combining optical trapping with fluorescence, absorption, electrochemical, and ablation methods. We have studied on microparticles, microdroplets, nanoparticles, polymers, supramolecules, micelles, amino acids and proteins in solution and reported their unique behavior. The remarkable achievement was performed in 2007 when the trapping laser was focused at solution surface. Various amino acids are crystalized, giving one single crystal at the position where trapping laser is focused and at the time when irradiated. This optical manipulation at solution surface is extended to dielectric and gold nanoparticles and to glass/solution interface. The initially trapped polystyrene nanoparticles at the focus form their periodical structure and scatter/propagate the trapping laser, shifting trapping site outside of the focus and forming a single large circle assembly. -

Cv- Hagan Bayley 2011
version of 09-09-11; printed: 12/10/11 CURRICULUM VITAE Hagan Bayley PRESENT ADDRESS: Department of Chemistry University of Oxford Chemistry Research Laboratory Mansfield Road Oxford, OX1 3TA, England, UK telephone: +44-1865-285101 fax: +44-1865-275708 email: [email protected] web site: bayley.chem.ox.ac.uk/ ACADEMIC CAREER: Professor of Chemical Biology, University of Oxford; Fellow of Hertford College (2003- present) Professor & Head, Dept. of Medical Biochemistry & Genetics, The Texas A&M University System Health Science Center (1997- 2003); Professor of Chemistry, Texas A&M University (1997- 2003); Full Member, Faculty of Genetics (1997- 2003) Principal Scientist, Worcester Foundation (1994-1996); Senior Scientist (1988- 1994); Associate Professor of Biochemistry & Molecular Biology (1991- 1996) and Physiology (1995- 1996), University of Massachusetts Medical Center; Associate Professor of Chemistry, Clark University (1996) Associate Professor, Center for Neurobiology & Behavior, Columbia University (1987-8); & Assistant Investigator, Howard Hughes Medical Institute, Columbia University (1985-8) University Lecturer in Organic Chemistry, Oxford University (1984-1985); & Fellow of Brasenose College, Oxford (1984-1985) Assistant Professor of Biochemistry, Columbia University (1981-1984) Postdoctoral Research (1979 - 1981) Massachusetts Institute of Technology Departments of Chemistry and Biology Laboratory of Professor H.G. Khorana Postgraduate (1974 - 1979) Harvard University Graduate School of Arts & Sciences Laboratory of Professor J.R. Knowles Ph.D in Chemistry (February 1979) Teaching Fellow (1974 - 1976) Undergraduate (1970 - 1974) Oxford University, Balliol College B.A. in Chemistry HONORS: 1970: Open scholarship, Oxford University; 1972: Distinction in Chemical Pharmacology, Oxford University; 1973: Herbertson Prize for Chemistry, Balliol College, Oxford; Gibbs Prize for Chemistry, (University Award for best final examination); 1974: B.A., Part II, First Class Honors; 1983: Irma T. -

Tim JONCKERS
1 ST ALPINE WINTER CONFERENCE ON MEDICINAL AND SYNTHETIC CHEMISTRYTim ST. ANTON, AUSTRIA | JANUARYJONCKERS 28 - FEBRUARY 1, 2018 JANSSEN PHARMACEUTICA Prof. Erick BOOK OF ABSTRACTS CONTENT SPEAKERS & ORAL COMMUNICATIONS - Biographies and Abstracts 3 POSTERS - Addressing Preclinical Toxicity – Approaches and Lessons Learned 70 POSTERS - Advances in Lead Generation 72 POSTERS - Advances in Synthetic Methods 75 POSTERS - Alternative Modalities 95 POSTERS - Challenges and Opportunities in Fragment Based Drug Discovery 98 POSTERS - Chemical Biology in Drug and Target Discovery 104 POSTERS - Drug Discovery Tales 121 POSTERS - Late Stage Functionalization 137 LIST OF ABSTRACTS 139 LIST OF AUTHORS 145 - 2 - SPEAKERS & ORAL COMMUNICATIONS Biographies and Abstracts - 3 - Erick M. CARREIRA ETH Zürich, Switzerland rof. Erick M. Carreira obtained a B.S. degree in 1984 from the University of Illinois at Urbana- PChampaign under the supervision of Scott E. Denmark and a Ph.D. degree in 1990 from Harvard University under the supervision of David A. Evans. After carrying out postdoctoral work with Peter Dervan at the California Institute of Technology through late 1992, he joined the faculty at the same institution as an assistant professor of chemistry and subsequently was promoted to the rank of associate professor in the Spring of 1996, and full professor in Spring 1997. Since September 1998, he has been professor of chemistry at the ETH Zürich in the Institute of Organic Chemistry. In 2011, he became associated with the Competence Center for Systems Physiology and Metabolic Diseases at ETH-Zürich. e is the recipient of numerous awards. Professor Carreira’s interests encompass several facets Hof chemical synthesis: natural products synthesis, chemistry as well as biology, catalysis, medicinal chemistry, and synthetic methods. -

Biologically Active Γ-Lactams: Synthesis and Natural Sources Cite This: Org
Organic & Biomolecular Chemistry REVIEW Biologically active γ-lactams: synthesis and natural sources Cite this: Org. Biomol. Chem., 2016, 14, 10134 J. Caruano,a,b G. G. Mucciolib and R. Robiette*a Received 22nd June 2016, The γ-lactam moiety is present in a large number of natural and non-natural biologically active com- Accepted 5th October 2016 pounds. The range of biological activities covered by these compounds is very broad. Functionalized DOI: 10.1039/c6ob01349j γ-lactams are thus of high interest and have great potential in medicinal chemistry. This review provides a www.rsc.org/obc description of the title compounds by focusing on their synthesis, natural sources and biological activities. 1 Introduction antibiotics. To overcome this resistance issue it was necessary to move away from the β-lactam core. Knowing that a suitable The γ-lactam ring, also known as γ-butyrolactam, pyrrolidin-2- activated amide bond is essential for the activity (but not the one, azolidin-2-one or 2-oxopyrrolidine, is part of the core β-lactam ring), the attention turned naturally to γ-lactams and structure of a large number of natural and non-natural com- their analogues, and more specifically to bicyclic γ-lactams pounds covering a broad spectrum of biological activities. such as penem derivatives. Accordingly, γ-lactams are of primary interest in medicinal It is in 1986 that the first γ-lactam analogues of penicillins chemistry and many synthetic strategies have been disclosed active as antibiotics were reported, independently, by Baldwin – to access this structural moiety. et al. and researchers from Eli Lilly.1 4 According to Baldwin, The interest in this scaffold started with the observation of who developed compound A (Fig. -

Chemistry Explored
Issue 4 // Autumn 2015 bristol.ac.uk/chemistry THE GREAT LEAP FORWARD HOW SELF-REPAIRING TECHNOLOGY DEVELOPED BY BRISTOL CHEMISTS COULD CHANGE THE WORLD DIAL A MOLECULE: HOW SYNTHESISING A MOLECULE JUST GOT EASIER LIFE IN CHEMISTRY: TAKE A VIRTUAL TOUR OF THE SCHOOL Welcome Also in this issue… Welcome to the autumn issue of Chemistry Explored. This summer saw us say goodbye to students who graduated in July and headed off to pursue their goals after completing their undergraduate and postgraduate degrees with us. You can read about some of the careers that chemistry News leads to in this edition. 2015 Science Alive 03 As well as celebrating academic Thornbury Festival, latest from Tyntesfield 04 success, we’ve attracted widespread I’m a scientist, get me out of here! 05 media attention. Research carried out Summer in the labs, Fresh ideas for first years 06 into advanced composite materials leading to the development of self- Features healing technology had a huge amount Molecules made easy 07 of press coverage, which you can read Generation next 08 more about in this edition. Technology, heal thyself 10 We’ve also welcomed Professor Life in Chemistry 12 Jonathan Clayden who has recently Climbing for charity 14 joined us from Manchester. We’re Infographic 15 delighted that Jonathan has chosen Life through a lens 16 to move to Bristol and there will be a feature about him and his research University of Bristol Production Editor Steve O’Brien Art Editor School of Chemistry Elaine Knight-Roberts Editorial Director Dan group in the next edition. -

Volume 40 – 2011
GOE SSMANNgazette A Publication of the Chemistry Department University of Massachusetts Amherst www.chem.umass.edu VOLUME 40 WINTER/SPRING 2011 INSIDE Chemistry Leads Development Alumni News ................................... .. 2 Points of Pride ....................................4 of Unique Integrated Science Curriculum Lab Notes ............................................ 4 Seminar Program .......................... 16 at UMass Amherst Senior Awards Dinner ................ 18 • • Degrees Awarded ......................... 18 A unique undergraduate science program was launched at UMass Amherst on January 18, 2011, Research Symposium .................. 19 marking the beginning of the Spring 2011 semester. The program, called iCons for Integrated Friends of Chemistry .................. 22 Letter from Head ......................... 24 Concentration in Science, will train students to synthesize and apply integrated scientific knowledge to solve society’s biggest problems in, e.g., renewable energy. UPCOMING EVENTS The complexity of the renewable energy problem requires collaborative, multidisciplinary solutions. Marvin Rausch Lectureship For example, the emerging biofuel industry will rely on teams of agronomists, chemists, chemical in Organometalic Chemistry engineers, plant biologists, microbiologists and others to bring new technologies from concept to Prof. Thomas E. Bitterwolf University of Idaho Getting to Know Our Newest April 14, 2011 Faculty Members Senior Awards Dinner INTERVIEW WITH PROFESSOR May 3, 2011 KEVIN KITTILSTVED (KK)