Biologically Active Γ-Lactams: Synthesis and Natural Sources Cite This: Org
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chiral Proton Catalysis: Design and Development of Enantioselective Aza-Henry and Diels-Alder Reactions
CHIRAL PROTON CATALYSIS: DESIGN AND DEVELOPMENT OF ENANTIOSELECTIVE AZA-HENRY AND DIELS-ALDER REACTIONS Ryan A. Yoder Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements for the degree Doctor of Philosophy in the Department of Chemistry, Indiana University June 2008 Accepted by the Graduate Faculty, Indiana University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Doctoral Committee _____________________________ Jeffrey N. Johnston, Ph.D. _____________________________ Daniel J. Mindiola, Ph.D. _____________________________ David R. Williams, Ph.D. _____________________________ Jeffrey Zaleski, Ph.D. April 24, 2008 ii © 2008 Ryan A. Yoder ALL RIGHTS RESERVED iii DEDICATION This work is dedicated to my parents, David and Doreen Yoder, and my sister, LeeAnna Loudermilk. Their unwavering love and support provided the inspiration for me to pursue my dreams. The sacrifices they have made and the strength they have shown continue to motivate me to be a better person each and every day. Thank you mom, dad, and little sis for being the rocks that I can lean on and the foundation that allowed me to find the happiness I have today. Without you, none of this would have been possible. iv ACKNOWLEDGEMENTS First and foremost I want to thank my research advisor, Professor Jeffrey N. Johnston. I am incredibly grateful for his mentoring and guidance throughout my time in the Johnston group. He has instilled in me a solid foundation in the fundamentals of organic chemistry and at the same time has taught me how to apply innovative and creative solutions to complex problems. -

Catalytic, Asymmetric Synthesis of Β-Lactams
J. Am. Chem. Soc. 2000, 122, 7831-7832 7831 Catalytic, Asymmetric Synthesis of â-Lactams Table 1. Diastereoselectivity in the Nucleophile-Catalyzed Reaction of Methylphenylketene 3b with Imine 2a Andrew E. Taggi, Ahmed M. Hafez, Harald Wack, Brandon Young, William J. Drury, III, and Thomas Lectka* Department of Chemistry Johns Hopkins UniVersity, 3400 North Charles Street Baltimore, Maryland 21218 ReceiVed May 22, 2000 The paramount importance of â-lactams (1) to pharmaceutical science and biochemistry has been well established.1 New chiral â-lactams are in demand as antibacterial agents, and their recent discovery as mechanism-based serine protease inhibitors2 has kept interest in their synthesis at a high level.3 However, most chiral methodology is auxiliary-based; only one catalytic, asymmetric synthesis of the â-lactam ring system is known, affording product a - ° in modest enantioselectivity.4 Many asymmetric auxiliary-based Reactions run with 10 mol% catalyst at 78 C and allowed to slowly warm to room temperature overnight. â-lactam syntheses rely on the reaction of imines with ketenes, a 3 process that generally proceeds without a catalyst. We recently catalyzed by 10 mol % bifunctional amine 4a gave a cis/trans dr accomplished a catalyzed reaction of imines and ketenes by of 3/97. A significant role for the H-bond contact in 4a is sug- making the imine component non-nucleophilic, as in electron- gested, as the sterically similar catalyst 4b, which does not contain deficient imino ester 2a.5 In this contribution, we report the ) 6 a hydrogen bond donor, gave low diastereoselectivity (dr 34/ catalytic, asymmetric reaction of imine 2a and ketenes 3 to 66). -

Regiocontrolled 1,3-Dipolar Cycloadditions of Nitrile Imines With
337 REGIOCONTROLLED 1,3-DIPOLAR CYCLOADDITIONS OF NITRILE IMINES WITH ACETYLENES AND ,-UNSATURATED SYSTEMS: SYNTHESIS OF POLYSUBSTITUTED AND RING FUSED PYRAZOLES WITH PHARMACEUTICAL ACTIVITY DOI: http://dx.medra.org/10.17374/targets.2017.20.337 Giulio Bertuzzi, Mariafrancesca Fochi and Mauro Comes Franchini Department of Industrial Chemistry “Toso Montanari”, University of Bologna, Viale Risorgimento 4, 40136 Bologna, Italy (e-mail: [email protected]) Abstract. 1,3-Dipolar cycloadditions of nitrile imines with functionalised acetylenes and ,-unsaturated systems of various sizes have been studied. After cycloaddition, mono- and ring-fused pyrazoles have been obtained. Computational studies allowed a theoretical description of the observed reactivity, in agreement with the experimentally observed regio-chemistry. Selected targeted structures for medicinal applications have been synthetized and in vitro cytotoxicity showed that these pyrazoles can be considered powerful lead compounds for further medicinally relevant targets development. Contents 1. Introduction 2. 1,3-Dipolar cycloaddition of nitrile imines with acetylenes 2.1. 1,3-Dipolar cycloaddition of nitrile imines with activated acetylenes: regiocontrolled synthesis of 4- and 5-substituted pyrazoles 2.2. 1,3-Dipolar cycloaddition with sulphur-based acetylenes: regiocontrolled synthesis of thieno[2,3- c]pyrazoles 2.3. 1,3-Dipolar cycloaddition with ynamide tert-butyl N-ethynyl-N-phenylcarbamate: experimental and theoretical investigation 3. 1,3-Dipolar cycloaddition of nitrile imines with cyclic dipolarophiles 3.1. 1,3-Dipolar cycloaddition of nitrile imines with α,β-unsaturated ketones: regiocontrolled synthesis of ring-fused pyrazoles 3.2. 1,3-Dipolar cycloaddition of nitrile imines with α,β-unsaturated lactones, thiolactones and lactams: regiocontrolled synthesis of ring-fused pyrazoles 4. -

The Development of the First Catalyzed Reaction of Ketenes and Imines: Catalytic, Asymmetric Synthesis of Â-Lactams Andrew E
Published on Web 00/00/0000 The Development of the First Catalyzed Reaction of Ketenes and Imines: Catalytic, Asymmetric Synthesis of â-Lactams Andrew E. Taggi, Ahmed M. Hafez, Harald Wack, Brandon Young, Dana Ferraris, and Thomas Lectka* Contribution from the Department of Chemistry, Johns Hopkins UniVersity, 3400 North Charles Street, Baltimore, Maryland 21218 Received February 5, 2002 Abstract: We report practical methodology for the catalytic, asymmetric synthesis of â-lactams resulting from the development of a catalyzed reaction of ketenes (or their derived zwitterionic enolates) and imines. The products of these asymmetric reactions can serve as precursors to a number of enzyme inhibitors and drug candidates as well as valuable synthetic intermediates. We present a detailed study of the mechanism of the â-lactam forming reaction with proton sponge as the stoichiometric base, including kinetics and isotopic labeling studies. Stereochemical models based on molecular mechanics (MM) calculations are also presented to account for the observed stereoregular sense of induction in our reactions and to provide a guidepost for the design of other catalyst systems. Introduction this structural motif a worthwhile goal for the synthetic organic 10 The clinical relevance of â-lactams continues to expand at a chemist, thus the synthesis of these nonantibiotic â-lactams surprising rate. Although their use as antibiotics is being will be the focus of this contribution. While considerable effort compromised to some extent by bacterial resistance pressures,1 has been put into synthetic methodology to construct the basic recently â-lactams (especially nonnatural ones) have achieved â-lactam skeleton, there have been few general methods many important nonantibiotic uses. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -

Pharmaceutical Composition Comprising Brivaracetam and Lacosamide with Synergistic Anticonvulsant Effect
(19) TZZ __T (11) EP 2 992 891 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 09.03.2016 Bulletin 2016/10 A61K 38/04 (2006.01) A61K 31/4015 (2006.01) A61P 25/08 (2006.01) (21) Application number: 15156237.8 (22) Date of filing: 15.06.2007 (84) Designated Contracting States: (71) Applicant: UCB Pharma GmbH AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 40789 Monheim (DE) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR (72) Inventor: STOEHR, Thomas 2400 Mol (BE) (30) Priority: 15.06.2006 US 813967 P 12.10.2006 EP 06021470 (74) Representative: Dressen, Frank 12.10.2006 EP 06021469 UCB Pharma GmbH 22.11.2006 EP 06024241 Alfred-Nobel-Strasse 10 40789 Monheim (DE) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: Remarks: 07764676.8 / 2 037 965 This application was filed on 24-02-2015 as a divisional application to the application mentioned under INID code 62. (54) PHARMACEUTICALCOMPOSITION COMPRISING BRIVARACETAM AND LACOSAMIDE WITH SYNERGISTIC ANTICONVULSANT EFFECT (57) The present invention is directed to a pharmaceutical composition comprising (a) lacosamide and (b) brivara- cetam for the prevention, alleviation or/and treatment of epileptic seizures. EP 2 992 891 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 992 891 A1 Description [0001] The present application claims the priorities of US 60/813.967 of 15 June 2006, EP 06 021 470.7 of 12 October 2006, EP 06 021 469.9 of 12 October 2006, and EP 06 024 241.9 of 22 November 2006, which are included herein by 5 reference. -

First Total Synthesis of (&Plus;)
The Journal of Antibiotics (2015) 68, 721–724 & 2015 Japan Antibiotics Research Association All rights reserved 0021-8820/15 www.nature.com/ja ORIGINAL ARTICLE First total synthesis of (+)-epogymnolactam, a novel autophagy inducer Yuji Okado, Kengo Shigetomi, Shinya Mitsuhashi and Makoto Ubukata A novel autophagy inducer, (+)-epogymnolactam (1), was first synthesized from cis-4-benzyloxy-2-butene-1-ol (2) in eight steps. A reliable preparation of optically pure epoxy alcohol (+)-3 from monobenzyl derivative (2) was established by a tandem strategy, Sharpless epoxidation/lipase kinetic resolution. The Journal of Antibiotics (2015) 68, 721–724; doi:10.1038/ja.2015.63; published online 27 May 2015 INTRODUCTION MATERIALS AND METHODS (+)-Epogymnolactam (1) was discovered as a novel autophagy inducer Chemicals of the highest commercial purity were used without further from a mycelial culture of Gymnopus sp. in our laboratory (Figure 1).1 purification. Thin-layer and silica gel column chromatography were performed Autophagy is one of the major intracellular degradation systems in by using Merck Silica Gel 60 F254 (Merck, Frankfurt, Germany) and Kanto Chemical Co. Silica Gel 60 N (spherical, neutral; Kanto Chemical Co., eukaryotic cells, eliminating damaged organelles and protein aggre- Tokyo, Japan), respectively. A DAICEL Chiralpak AD-H column (φ 0.64 × 25 gates to maintain cytoplasmic homeostasis. This degradation pathway cm2; DAICEL, Osaka, Japan) and a Waters 600 System (Waters, Milford, MA, has important roles in such diseases as cancer, neurodegenerative and USA) were used for chiral HPLC. 1Hand13C NMR spectra were recorded infectious diseases. Thus, the application of autophagy inducer would using a JEOL JNM EX-270 FT-NMR (JEOL, Tokyo, Japan), and HSQC and help to understand the regulatory roles of autophagy in human HMBC spectra were measured with a Bruker AMX-500 (Bruker, Billerica, diseases, and provide insight into the development of therapeutic MA, USA). -

Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001
CONFERENCE REPORT 732 CHIMIA 2001, 55, No.9 CONFERENCE REPORT Chimia 55 (2001) 732-736 © Schweizerische Chemische Gesellschaft ISSN 0009-4293 Selective Synthesis: New Reagents for Specific Transformations Champery, June 6-9, 2001 Christian G. Bochet* Keywords: CERC3 . Champery . European chemistry' Organic synthesis· Workshop The chairmen of the European Re- from the chiral centers, had a strong im- The day was concluded with the first search Councils' Chemistry Committees pact on the geometry of the dimeric cata- plenary lecture, by Professor Varinder (CERC3) elected organic synthesis as lyst; these effects could be so strong that Aggarwal (University of Bristol, UK). this year's topic for their annual work- similar catalysts varying only by one sub- He explained the asymmetric epoxida- shop, and Switzerland as the host coun- stituent gave opposite enantiomers [1]. tion reaction, in which, instead of using try. The conference chairman, Prof. The afternoon session was concluded the classical strategy (i.e. oxidation of an Philippe Renaud made the excellent by Petri Pihko (The Scripps Research In- alkene), the key step is a carbon-carbon choice of Champery for hosting the stitute, La Jolla, USA), on the synthesis bond forming process (the so-called Co- workshop; the combination of his enthu- of Azaspiracid (Fig. 1), a very potent tox- rey-Chaykovsky epoxidation). The beau- siasm, efficient organization and the in, isolated from Irish mussels. The FGHI ty of this reaction, very simple in its ex- beautiful alpine scenery made this meet- spirocyclic portion proved to be a very perimental realization, is revealed by the ing a really unique event. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -
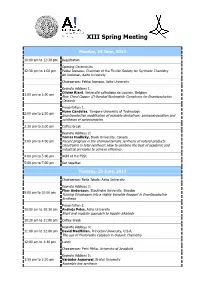
XIII Spring Meeting
XIII Spring Meeting Monday, 24 June, 2013 10:00 am to 12:30 pm Registration Opening Ceremonies 12:30 pm to 1:00 pm Pekka Joensuu, Chairman of the Finnish Society for Synthetic Chemistry Ari Koskinen, Aalto University Chairperson: Pekka Joensuu, Aalto University Keynote Address 1: Olivier Riant, Université catholique de Louvain, Belgium 1:00 pm to 2:00 pm New Chiral Copper (I)-Bonded Nucleophile Complexes for Enantioselective Catalysis Presentation 1: Nuno Candeias, Tampere University of Technology 2:00 pm to 2:30 pm Enantioselective modification of oxindole derivatives: aminooxygenation and syntheses of spirooxindoles 2:30 pm to 3:00 pm Coffee break Keynote Address 2: Tomas Hudlicky, Brock University, Canada 3:00 pm to 4:00 pm Recent progress in the chemoenzymatic synthesis of natural products. Constraints in total synthesis: How to combine the best of academic and industrial principles to achieve efficiency. 4:00 pm to 5:00 pm AGM of the FSSC 5:00 pm to 7:00 pm Get together Tuesday, 25 June, 2013 Chairperson: Reija Jokela, Aalto University Keynote Address 3: Pher Andersson, Stockholm University, Sweden 9:00 am to 10:00 am Turning Dihydrogen into a Highly Versatile Reagent in Enantioselective Synthesis Presentation 2: 10:00 am to 10:30 am Andrejs Pelss, Aalto University Short and modular approach to lepadin alkaloids 10:30 am to 11:00 am Coffee Break Keynote Address 4: 11:00 am to 12:00 am David MacMillan, Princeton University, U.S.A. The use of Photoredox Catalysis in Organic Chemistry 12:00 am to 1:30 pm Lunch Chairperson: Petri Pihko, -

FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr
FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr. Lee D. Arnold 1987, Ph.D. Serine β-Lactones in Syntheses of Amino Acids None President & CEO of Discovery Elucidations [email protected] Dr. Hanaa Assil 1989, M.Sc. Solid Supports for Azodicarboxylates in Mitsunobu Reactions n/a Unknown Unknown and Synthesis of Amino Acids Dr. Karine Auclair 1999, Ph.D. Biosynthetic Studies on the Polyketide Lovastatin: Enzyme- Prof. P. Ortiz De Montellano, Department Assoc. Professor, Department of Chemistry, McGill University, [email protected] Catalyzed Diels-Alder Reactions of Pharmaceutical Chemistry, University of Montreal, Que., Canada California at San Francisco, USA Dr. Jennifer Blunston (nee Caplan) 2001, Ph.D. Diaminopimelic Acid Analogues as Inhibitors of Enzymes Prof. D. Waisman, Departments of Stay-at-home mom, raising son Rhys. Previously Jennifer [email protected] Involved in Bacterial Lysine Biosynthesis Biochemistry and Oncology, University of Blunston was Assistant Professor of Chemistry, St. Mary's Calgary, Canada University College, Calgary, AB, Canada Dr. Marc Boudreau 2007, Ph.D. Nucleoside Dicarboxylates as Mimics of Diphosphates and Prof. Hagan Bayley, Oxford University Postdoc with Professor Shahriar Mobashery, University of Notre [email protected] Disulfide Bond Replacement in Pediocin PA1 Dame Dr. Doug Burr 2006, Ph.D. Studies of the in vitro Activity of Lovastatin Nonaketide Prof. David Sherman, Dept. Medicinal Research scientist at Perkin Elmer, Boston, Massachusetts [email protected] Synthase Chemistry, University of Michigan, Ann Arbor, MI Dr. Hengmiao (Henry) Cheng 1992, Ph.D. Mechanism and Inhibition of Peptidylglycine α-Hydroxlating Prof. E.J. Corey, Department of Chemistry, Senior Research Investigator, Pfizer Global Research and [email protected] Monooxygenase Harvard University Development, Pfizer Inc., Groton, CT, USA Dr. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0