Geochemical Pattern of Soils in Bobovdol Valley, Bulgaria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Company Profile
www.ecobulpack.com COMPANY PROFILE KEEP BULGARIA CLEAN FOR THE CHILDREN! PHILIPPE ROMBAUT Chairman of the Board of Directors of ECOBULPACK Executive Director of AGROPOLYCHIM JSC-Devnia e, ECOBULPACK are dedicated to keeping clean the environment of the country we live Wand raise our children in. This is why we rely on good partnerships with the State and Municipal Authorities, as well as the responsible business managers who have supported our efforts from the very beginning of our activity. Because all together we believe in the cause: “Keep Bulgaria clean for the children!” VIDIO VIDEV Executive Director of ECOBULPACK Executive Director of NIVA JSC-Kostinbrod,VIDONA JSC-Yambol t ECOBULPACK we guarantee the balance of interests between the companies releasing A packed goods on the market, on one hand, and the companies collecting and recycling waste, on the other. Thus we manage waste throughout its course - from generation to recycling. The funds ECOBULPACK accumulates are invested in the establishment of sustainable municipal separate waste collection systems following established European models with proven efficiency. DIMITAR ZOROV Executive Director of ECOBULPACK Owner of “PARSHEVITSA” Dairy Products ince the establishment of the company we have relied on the principles of democracy as Swell as on an open and fair strategy. We welcome new shareholders. We offer the business an alternative in fulfilling its obligations to utilize packaged waste, while meeting national legislative requirements. We achieve shared responsibilities and reduce companies’ product- packaging fees. MILEN DIMITROV Procurator of ECOBULPACK s a result of our joint efforts and the professionalism of our work, we managed to turn AECOBULPACK JSC into the largest organization utilizing packaging waste, which so far have gained the confidence of more than 3 500 companies operating in the country. -

Sofia Model”: Creation out of Chaos
The “Sofia Model”: Creation out of chaos Pathways to creative and knowledge-based regions ISBN 978-90-75246-62-9 Printed in the Netherlands by Xerox Service Center, Amsterdam Edition: 2007 Cartography lay-out and cover: Puikang Chan, AMIDSt, University of Amsterdam All publications in this series are published on the ACRE-website http://www2.fmg.uva.nl/acre and most are available on paper at: Dr. Olga Gritsai, ACRE project manager University of Amsterdam Amsterdam institute for Metropolitan and International Development Studies (AMIDSt) Department of Geography, Planning and International Development Studies Nieuwe Prinsengracht 130 NL-1018 VZ Amsterdam The Netherlands Tel. +31 20 525 4044 +31 23 528 2955 Fax +31 20 525 4051 E-mail: [email protected] Copyright © Amsterdam institute for Metropolitan and International Development Studies (AMIDSt), University of Amsterdam 2007. All rights reserved. No part of this publication can be reproduced in any form, by print or photo print, microfilm or any other means, without written permission from the publisher. The “Sofia Model”: Creation out of chaos Pathways to creative and knowledge-based regions ACRE report 2.10 Evgenii Dainov Ivan Nachev Maria Pancheva Vasil Garnizov Accommodating Creative Knowledge – Competitiveness of European Metropolitan Regions within the Enlarged Union Amsterdam 2007 AMIDSt, University of Amsterdam ACRE ACRE is the acronym for the international research project Accommodating Creative Knowledge – Competitiveness of European Metropolitan Regions within the enlarged Union. The project is funded under the priority 7 ‘Citizens and Governance in a knowledge-based society within the Sixth Framework Programme of the EU (contract no. 028270). Coordination: Prof. -

Bulgarianproperties
Offer: Development land for private house in Bankya in Bankya Ref. No.: Bo 274 URL address of the offer: https://www.bulgarianproperties.com/41428 Development land for private house in Bankya Price € 110 000 € 65 000 The price has been reduced by € 45 000 (40.91%) Location: Bankya For sale Type of property: Development land Area features : In town. , In mountain, In vacation place, In balneological resort, In town Area: 1000.00 m2 Garden: no Condition: read text Authorised agency Responsible agent Sergey Pelovski Sofia Mobile: +359 882 817 459 Phone: +359 2 425 68 21 Address: 22, Zlaten Rog Str., floor 4, office 7, Sofia 1407 Skype: bulgarianproperties.com Land in regulation on asphalt road with beautiful panorama, 14 km from the capital View our new offer for a plot of development land (1000 sq.m.), located on Zahari Stoyanov Str. in the town of Bankya, set 14 km from the capital Sofia. The plot has quiet location only 1 km from the center of the SPA resort. It opens spacious and fantastic panoramic view, to the east, south and west, towards the horizon and the mountains Vitosha and Lyulin. Its entire left border (about 60 m), has an already built, 3 m high concrete fence. The plot is suitable for the construction of a private house with the following parameters: Density - 30%; Intensity - 1; Maximum height - 10 m. The plot is located on an accessible road (22 meters southern face at the asphalt street with water and sewerage Page 1 Offer: Development land for private house in Bankya in Bankya Ref. -

Forestry: Bridge to the Future” Is Financially Supported By: Ministry of Agriculture, Food and Forestry Mondi LTD of Bulgaria;
Book of Abstracts FORESTRY Bridge to the Future International Conference, 5–8 May, 2021, Sofia, Bulgaria The International Scientific Conference “Forestry: Bridge to the Future” is financially supported by: Ministry of Agriculture, Food and Forestry Mondi LTD of Bulgaria; Northwestern State Forestry Enterprise, Vraca Andreas Stihl, Bulgaria North Central State Forestry Enterprise, Gabrovo National Association of Owners of Non-State Forests “Gorovladeletz” Northeastern State Forestry Enterprise, Shumen Southwestern State Forestry Enterprise, Blagoevgrad South Central State Forestry Enterprise, Smolyan Southeastern State Forestry Enterprise, Sliven The International Scientific Conference “Forestry: Bridge to the Future” is organizing supported by: Editors: Marius Dimitrov, Svetoslav Anev, Stanimir Stoilov Pre-pres: Svetoslav Anev Cover design: Svetoslav Anev University of Forestry, Sofia, Bulgaria https://ltu.bg/ Ysabeau Infant; Vollkorn ISBN: 978-954-332-183-4 Book of Abstracts FORESTRY Bridge to the Future International Conference, 5–8 May, 2021, Sofia, Bulgaria Organizing Committee International Scientific Committee Honorable Chairman: Prof. DSc. Ivan ILIEV Chair: Marius DIMITROV – University of Forestry, Sofia, Bulgaria – Rector of the University of Forestry, Sofia, Vice-chair: Nasko ILIEV – University of Forestry, Sofia, Bulgaria Bulgaria Secretary: Momchil PANAYOTOV – University of Forestry, Sofia, Bulgaria Members: Chairman: Assoc. prof. Dr. Marius DIMITROV Alexandar TASHEV – University of Forestry, Sofia, Bulgaria – Dean of the -

GIS-Sofia Data Base
October 26-29, 2015, Yucatan, Mexico GIS-Sofi a Data Base Vanya Petrova GIS-Sofi a Ltd., Sofi a, Bulgaria Founded seven thousand years ago, Sofi a is the location, boundaries and features of the property. second oldest city in Europe. It has been given In the family of geographical information several names in the course of history and the system one of the main positions holds remains of the old Thracian and Roman cities can photogrammetry — the fastest and the most still be seen today. The city became known as Sofi a modern way to acquire uptodate information of from the beginning of the 15th century. In 1879 the Earth’s surface. Photogrammetry Department Sofi a became capital of Bulgaria. at GIS SOFIA applies digital photogrammetric Nowadays the territory of Sofi a Municipality is software PHOTOMOD in projects based on aerial 1349 sq. km and includes the areas of 4 towns — City and satellite images. During the years 20002013 of Sofi a (around 200 sq. km), three other towns: different kind of sources were used — analogue Bankya, Novi Iskar, Buhovo and 34 villages. The fi lm cameras (RMK A 15/23, RMK TOP 30), City of Sofi a with 1.3 million population includes digital cameras (DMC, UltraCamXp), various high 24 administrative and territorial districts. resolution satellite sensors (IKONOS, QuickBird, The main function of creation and maintenance KOMPSAT, GeoEye1). of the cadastral information system of Sofi a The fi rst of its kind, in Bulgaria, orthophotoatlas of Municipality is in charge of GIS SOFIA Ltd. Sofi a Municipality at a scale of 1:5000 was printed, (Geographical Information System – Sofi a), using aerial images with 10 cm GSD — a luxurious, established in 1999, as a Sofi a Municipality limited edition. -

Spa Resorts in Bulgaria, Italy, Lithuania, Poland, Portugal and Turkey
SPA RESORTS IN BULGARIA, ITALY, LITHUANIA, POLAND, PORTUGAL AND TURKEY 1 COORDINATOR Letets Hristo Toprakchiev Secondary school http://soubozhurishte.com/news.php Bulgaria, Bozhurishte, 10 „Ivan Vazov“ street PARTNERS Liceo Statale „ E. Majorana“ https://www.majoranaliceo.gov.it/scuola/ Italy, San Giovanni La Punta,Via G. Motta, 87 Agrupamento de Escolas do Forte da Casa http://portal.aefc.edu.pt/ Portugal,Forte da Casa, rua Rua Da Republica 2625-503 Siauliai Ragaine progymnasium http://ragaine.su.lt/ Lithuania, Siauliai, Tilzes street 85 Publiczne Gimnazjum Nr 26 Im. M. Reja http://www.gim26.edu.pl/ Poland, Lodz, al. 1 MAJA 89 Ted Malatya http://www.tedmalatya.com/ Turkey, Inonu Universitesi Kampusu Elazig Yolu uzeri 10. Km 2 Water in Our World WOW SPA RESORTS IN BULGARIA, ITALY, LITHUANIA, POLAND, PORTUGAL AND TURKEY TRAVEL GUIDE Средно училище „Летец Христо Топракчиев“ Божурище, 2018 3 Water in Our World WOW SPA RESORTS in Bulgaria, Italy, Lithuania, Poland, Portugal and Turkey TRAVEL GUIDE Authors Bulgaria Tatyana Dimitrova, Teodora Taneva, Lyubomira Stoyanova Italy Giovanna Cantone, Andrea Cosentino Lithuania Lijana Jurgeliene, Klelija Rakstyte Poland Agnieszka Duda, Beata Oleksiewicz Portugal Helena Ramos, Maria João Valério, Maria da Luz Amado Turkey Eylem İÇER, Serkan Korkmaz 4 INTRODUCTION The Travel guide of the spa resorts is suitable and useful for schools, librar- ies, travel agencies and institutions working in the field of education and tourism. Schools could use the guide in their English and Geography lessons, in project based classes or extra curriculum activities. Libraries, as information centers, could pro- vide information to their readers and communities. Since there isn’t available infor- mation concerning some of the resorts in native languages on the Internet, this Guide would be a good instrument for Travel agencies to promote the spa resorts. -
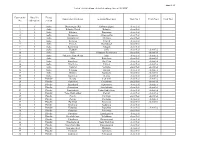
Annex 14 Consecutive No No of the Railway Line Energy Section from Station/Block Post to Station/Block Post Track No 1 Track No
Annex 14 List of electrified/non-electrified railway lines of SE NRIC Consecutive No of the Energy from station/block post to station/block post Track No 1 Track No 2 Track No 3 No railway line section 1 1 Sofia Dimitrovgrad RS Kalotina Zapad electrified 2 1 Sofia Kalotina Zapad Kalotina electrified 3 1 Sofia Kalotina Dragoman electrified 4 1 Sofia Dragoman Aldomirovtsi electrified 5 1 Sofia Aldomirovtsi Slivnitsa electrified 6 1 Sofia Slivnitsa Petarch electrified 7 1 Sofia Petarch Kostinbrod electrified 8 1 Sofia Kostinbrod Voluyak electrified 9 1 Sofia Voluyak Sofia electrified electrified 10 1 Sofia Sofia Poduyane Patnicheska electrified electrified 11 1 Sofia Poduyane Patnicheska Iskar electrified electrified 12 1 Sofia Iskar Kazichene electrified electrified 13 1 Sofia Kazichene Elin Pelin electrified electrified 14 1 Sofia Elin Pelin Vakarel electrified electrified 15 1 Sofia Vakarel Verinsko electrified electrified 16 1 Sofia Verinsko Ihtiman electrified electrified 17 1 Sofia Ihtiman Kostenets electrified electrified 18 1 Sofia Kostenets Belovo electrified electrified 19 1 Plovdiv Belovo Septemvri electrified electrified 20 1 Plovdiv Septemvri Pazardzhik electrified electrified 21 1 Plovdiv Pazardzhik Ognyanovo electrified electrified 22 1 Plovdiv Ognyanovo Stamboliyski electrified electrified 23 1 Plovdiv Stamboliyski Todor Kableshkov electrified electrified 24 1 Plovdiv Todor Kableshkov Plovdiv electrified electrified 25 1 Plovdiv Plovdiv Por Iztok electrified electrified 26 1 Plovdiv Plovdiv Por Iztok electrified electrified -

Municipal Housing Policies: a Key Factor for Successful Integration at the Local Level
MUNICIPAL HOUSING POLICIES: A KEY FACTOR FOR SUCCESSFUL INTEGRATION AT THE LOCAL LEVEL Snezhina Gabova Sofia, 2020 Municipal Housing Policies: a Key Factor for Integration at the Local Level This study was financed by the Representation of the UN High Commissioner for Refugees (UNHCR) in Bulgaria. The views and recommendations presented in this document express only the author’s opinion and might not reflect the official position of UNHCR. About the author: Snezhina Gabova is a researcher at Sofia Development Association. She has a long-standing experience in research and project development in the fields of education, good governance, integration of third-country nationals. She holds an MA in Philosophy from Sofia University St. Kliment Ohridski and PhD in Philosophy from Villanova University in Pennsylvania, USA. Cover photo: Nikolay Stoykov, 2015 1 Municipal Housing Policies: a Key Factor for Integration at the Local Level Abbreviations AMIF Asylum, Migration and Integration Fund ASA Agency for Social Assistance BAS Bulgarian Academy of Sciences BCRA Bulgarian Cities and Regions Association BCRM Bulgarian Council on Refugees and Migrants BHC Bulgarian Helsinki Committee BIPs Beneficiaries of international protection BP Border Police BRC Bulgarian Red Cross CEMR Council of European Municipalities and Regions CoM Council of Ministers CRA Civil Registration Act DG Directorate General EC European Commission ESIF European Structural and Investment Funds EU European Union FAR Foundation for Access to Rights IOM International Organization -

Administrative Division of Sofia Municipality and Large Cities Act
Administrative Division of Sofia Municipality and Large Cities Act Promulgated, SG No. 66/25.07.1995, amended and supplemented, SG No. 80/8.09.1995, amended, SG No. 90/15.10.1999, SG No. 31/10.04.2018 Text in Bulgarian: Закон за териториалното деление на столичната община и големите градове Article 1. (1) This act prescribes the administrative division of the capital municipality and of the cities with a population of over 300 000 people. (2) Within the meaning of this act cities with population of over 300 000 people are Plovdiv and Varna. Article 2. (1) The capital municipality is divided into quarters and mayoralties in the quarters. (2) Established in the capital municipality are twenty four quarters with the following names and boundaries: 1. "Sredets" quarter with boundaries: the Largo, Tsar Osvoboditel Blvd., Knyaz Alexander Battenberg sq, Moskovska Str., Paris Str., Shipka Str., Khan Omourtag Str., Sitnyakovo Blvd., Peyu K. Yavorov Blvd., Dragan Tsankov Blvd., Evlogy Georguiev Blvd., Fridtjof Nansen Str., Patriarch Evtimiy Blvd. Vitosha Blvd. and St. Nedelya sq.; 2. "Krasno selo" quarter with boundaries: Hristo Botev Blvd., Prague Blvd., Georgui Sofiysky Str. Dimitar Nestorov Str., Bulgaria Blvd., Todor Kableshkov Str., Ovcha Koupel Blvd., Vladayska Reka, Zhitnitsa Str., Gornobansky Blvd., the North-eastern boundary of the tram depot, Vladayska reka, railway, Dobrudzhansky kray Str., Marko Balabanov Str, Pozitano Str.; 3. "Vazrazhdane" quarter with boundaries: Cyril and Methodius Str., Slivnitsa Blvd., Maria-Louisa Blvd., St. Nedelya sq., Pozitano Str., Marko Balabanov Str., Dobrudzhansky kray Str., railway, Vladayska river, Konstantin Velichkov Blvd.; 4. "Oborishte" quarter with boundaries: Slivnitsa Blvd., Danail Nikolaev Blvd., Sitnyakovo Blvd., Khan Omurtag Str., Shipka Str., Paris Str, Moskovska Str., Knyaz Alexander Battenberg sq., The Largo and Maria- Louisa Blvd.; 5. -

Verkaufspunkte Vignette Bulgarien
Verkaufspunkte Vignette Bulgarien AKZ Nr. Name Straße PLZ + Ort 16 15 255 PETROL-Station Lomsko Chaussee 226 1000 Sofia 16 15 256 PETROL-Station Pencho Slaveykov Street, Serdika Residential Area 1000 Sofia 16 15 258 PETROL-Station Konstantin Velichkov Boulevard 1000 Sofia 16 15 259 PETROL-Station Lyulin Residential Area 5 1000 Sofia 16 15 260 PETROL-Station Exit to Dragoman 1000 Sofia 16 15 261 PETROL-Station Iliensko Chaussee 1000 Sofia 16 15 262 PETROL-Station Bozhur Motel, Ringroad 1000 Sofia 16 15 263 PETROL-Station Iztok Motel, Ringroad 1000 Sofia 16 15 264 PETROL-Station Yordan Iliev Street 3, Maldost Residential Area 1000 Sofia 16 15 265 PETROL-Station Tzar Boris III Boulevard 17, Pavlovo District 1000 Sofia 16 15 268 PETROL-Station Nikola Vaptsarov Boulevard 4 1000 Sofia 16 15 269 PETROL-Station Dragomansko Chaussee, Milevo Hanche 1000 Sofia 16 15 270 PETROL-Station Gorublyane District 1000 Sofia 16 15 271 PETROL-Station Botevgradsko Chaussee, Ringroad, Vrazhdebna Distri 1000 Sofia 16 15 272 PETROL-Station Dianabad District, Vasil Kalchev District 1000 Sofia 16 15 273 PETROL-Station 2 Mladost Residential Area 1000 Sofia 16 15 274 PETROL-Station Bulina livada Street, Gevgeliiski District 1000 Sofia 16 15 275 PETROL-Station M. Kusevich Street 1, Kransna Polyana District 1000 Sofia 16 15 276 PETROL-Station Obelya Residential Area 1000 Sofia 16 15 277 PETROL-Station 1st Balgarska Street, Orlandovtsi District 1000 Sofia 16 15 278 PETROL-Station Lomsko Chaussee, Ringroad 1000 Sofia 16 15 279 PETROL-Station Asen Yordanov Street, Junction -

Population Dynamics and Land Cover Changes of Urban Areas
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RAUmPlan - Repository of Architecture, Urbanism and Planning SPATIUM International Review UDC 711.4:314.114(497.11) ; No. 31, July 2014, pp. 22-29 711.4:314.114(450) ; 711.4:314.114(497.2) -7 Original scientific paper DOI: 10.2298/SPAT1431022K POPULATION DYNAMICS AND LAND COVER CHANGES OF URBAN AREAS Nikola Krunić1,2,3, Institute of Architecture and Spatial & Urban Planning of Serbia, Belgrade, Serbia Marija Maksin3, Institute of Architecture and Spatial & Urban Planning of Serbia, Belgrade, Serbia Saša Milijić3, Institute of Architecture and Spatial & Urban Planning of Serbia, Belgrade, Serbia Olgica Bakić3, Institute of Architecture and Spatial & Urban Planning of Serbia, Belgrade, Serbia Jasmina Đurđević3, Institute of Architecture and Spatial & Urban Planning of Serbia, Belgrade, Serbia In order to enable efficient management of spatial development of cities, it is essential to analyse changes in land cover, in the ‘consumption’ of the land surrounding cities and the attained rationality with respect to the use of already urban land (reflected in the urban population density). This paper provides an overview of the land cover changes in the period between 1990 and 2006, and the potential correlation between the dynamics of the total population change on the one hand, and the land cover change on the other. The initial hypotheses of this paper are: (1) occupation and sealing of productive soil in peri-urban zones is not proportional to the population dynamics of cities and their metropolitan areas; and (2) expansion of soil sealing in peri-urban zones is not significantly affected by the differences with regard to the natural surroundings and historical development of cities, nor by these cities being developed cities or cities in transition, capitalistic or post-socialist cities, etc. -

Fulbright Newsletter No. 63 October
Bulgarian-American Commission for Educational Exchange No 63 www.fulbright.bg Newsletter October-December 2010 Sofia 1000, Al. Stamboliiski blvd., tel. (359 2) 981 85 67, 980 82 12, 981 68 30; fax (359 2) 988 45 17; E-mail: [email protected]; Internet: www.fulbright.bg, www.fisi-bg.info Final Nominations for Bulgarian Fulbright Grantees in AY 2011-2012 his year the Commission received 49 applications for Fulbright Tsenior scholar and graduate study grants, Hubert Humphrey fel- lowships and non-degree grants for doctoral students. The bination- al reviewing committees recommended 28 students and 13 scholars for interview. The interviews were conducted on June 28 and 29 and on July 1, 2010. The applicants represented a wide variety of fields and were well qualified and motivated. The following candidates for Fulbright scholarships and Hubert H. Humphrey fellowships in AY 2011-12 were nominated by the Commission Board: Senior Scholars Principal candidates: 1. Plamen Makariev – philosophy 2. Stoycho Metodiev – animal breeding Fulbright Interview, June-July, 2010. Members of the nomination committee (from left 3. Violina Rizova – biogeochemistry to right): Randall Baker, Fulbright Board Member; Dr. Julia Stefanova, Executive Director; Ken 4. Tsanka Dikova – dental medicine Moskowitz, Fulbright Board Member, PAO, U.S. Embassy 5. Dimitar Antonov – environmental science Alternate candidate: Alternate candidates: 1. Petya Koprinkova-Hristova – robotics 1. Irina Simova – comparative literature 2. Anthony Arguirov – film directing Graduate Students – Degree Programs 3. Veselka Petrova – law Principal candidates: 1. Milen Markov – business administration Fulbright-Oklahoma Grant 2. Mariya Miteva – electronic commerce Principal candidate: 3. Marina Petrova – graphic design 1. Edouard Shahpazyan – business administration 4.