The Marine Algæ of Denmark Contributions to Their Natural History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hele Rapporten I Pdf Format
Miljø- og Energiministeriet Danmarks Miljøundersøgelser Typeinddeling og kvalitetselementer for marine områder i Danmark Vandrammedirektiv–projekt, Fase 1 Faglig rapport fra DMU, nr. 369 [Tom side] Miljø- og Energiministeriet Danmarks Miljøundersøgelser Typeinddeling og kvalitetselementer for marine områder i Danmark Vandrammedirektiv–projekt, Fase 1 Faglig rapport fra DMU, nr. 369 2001 Kurt Nielsen Afdeling for Sø- og Fjordøkologi Bent Sømod Aarhus Amt Trine Christiansen Afdeling for Havmiljø Datablad Titel: Typeinddeling og kvalitetselementer for marine områder i Danmark Undertitel: Vandrammedirektiv-projekt, Fase 1 Forfattere: Kurt Nielsen1, Bent Sømod2, Trine Christiansen3 Afdelinger: 1Afd. for Sø- og Fjordøkologi 2Aarhus Amt 3Afd. for Havmiljø Serietitel og nummer: Faglig rapport fra DMU nr. 369 Udgiver: Miljø- og Energiministeriet Danmarks Miljøundersøgelser URL: http://www.dmu.dk Udgivelsestidspunkt: August 2001 Faglig kommentering: Dorte Krause-Jensen, Danmarks Miljøundersøgelser; Henning Karup, Miljøstyrelsen; Nanna Rask, Fyns Amt Layout: Pia Nygård Christensen Korrektur: Aase Dyhl Hansen og Pia Nygård Christensen Bedes citeret: Nielsen, K., Sømod, B. & T. Christiansen 2001: Typeinddeling og kvalitetselementer for marine områder i Danmark. Vandrammedirektiv-projekt, Fase 1. Danmarks Miljøundersøgelser. 107 s. - Faglig rapport fra DMU nr. 369. http://faglige-rapporter.dmu.dk. Gengivelse tilladt med tydelig kildeangivelse. Sammenfatning: Rapporten indeholder en opdeling af de danske kystområder i 16 forskellige typer i henhold -

Habitat Suitability for Juvenile Flatfish of the Inner Danish Waters Section for Ecosystem Based Marine Management
Downloaded from orbit.dtu.dk on: Oct 07, 2021 Habitat Suitability for Juvenile Flatfish of the Inner Danish Waters Section for Ecosystem Based Marine Management Brown, Elliot John Publication date: 2019 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Brown, E. J. (2019). Habitat Suitability for Juvenile Flatfish of the Inner Danish Waters: Section for Ecosystem Based Marine Management. Technical University of Denmark. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Habitat Suitability for Juvenile Flatfish of the Inner Danish Waters Section for Ecosystem Based Marine Management Elliot John Brown PhD Thesis Supervisor: Josianne G. Støttrup Advisor: Ulf Bergström Preface This thesis was submitted in partial fulfilment of the requirements for the degree Doctor of Philosophy (PhD) at the Technical University of Denmark. The research presented in this thesis was carried out under the supervision of Senior Researcher Josianne Støttrup within the Section for Ecosystem Based Management of the National Institute of Aquatic Resources (DTU Aqua). -

Varde Anholt Varde Bornholm Varde Fur Varde Langeland Varde Femų
Varde Anholt Varde Bornholm Varde Fur Varde Langeland Varde Femø 2. edition edition 22.09.20172. UK Varde Bogø Installation— and User guide Revision 2 Soldalen 12, 7100 Vejle, Danmark, Tel. +45 7482 0003. vardeovne.dk 1 Congratulation on purchasing your new stove Varde Ovne A/S is a Danish company specializing in functional, environmentally friendly and designed quality stoves. Index Welcome and index Page 2 Technical specifications Anholt Page 3 Technical specifications Bornholm Page 4 Technical specifications Fur Page 5 Technical specifications Langeland Page 6 Technical specifications Femø Page 7 Technical specifications Bogø Page 8 Regulations and approval Page 9 Flooring and distance Page 10 Installations distances Page 11 Chimney Page 12 Air supply Page 13 How to light and stoke a fire Page 14 How to light and stoke a fire Page 15 Operation Page 16 Vermiculit e Page 17 How to choose the wood Page 18 Maintenance Page 19 Troubleshooting Page 20 Spare parts Page 21 Testcertificate (RRF) Page 22 Warranty Page 23 2 Technical Specification Anholt Model Anholt Height (mm) 1005 Wide (mm) 458 Depth (mm) 352 Weight (kg) 85 Effect 3-7 kW Nominal Output 5,5 kW Heated area 30-105m² Efficiency 80 % EEI 107 Flue gas data 273°C at 25°C, 12Pa. Combustion Chamber : (H x W x D): 245-360/300/280mm Flue outlet: Ø 15cm (Mounting height top: 99cm) Distance to non inflammable: 5-10cm (Recommended) Distance to inflammable wall and materiels: Rear = 30cm, Sides = 45cm, In front = 110cm 3 Technical Specification Bornholm Model Bornholm Height (mm) 1005 Wide (mm) 458 Depth (mm) 352 Weight (kg) 85 Effect 3-7 kW Nominal Output 5,5 kW Heated area 30-105m² Efficiency 80 % EEI 107 Flue gas data 273°C at 25°C, 12Pa. -
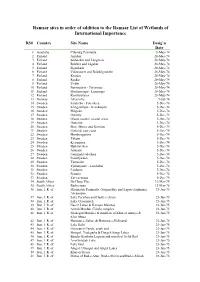
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Funen Energy Plan
STRATEGIC ENERGY PLANNING AT MUNICIPAL LEVEL Funen Energy Plan Funen is characterised by a high share of agriculture, From a systemic point of view, he emphasizes that the with remarkable biomass resources, and well- energy system needs to be redesigned. He finds it developed district heating and gas distributing important to link large heat pumps to surplus heat systems. To ensure the success and stability of future from industries so that they will be as cost-effective as local investments in the energy sector, Funen has possible. Furthermore, he stresses the importance of developed a political framework for the future energy using heat pumps to harness production of electricity investments, an energy plan. from wind turbines. The plan was developed in a wide cooperation between 9 municipalities on Funen and Ærø, 5 supply companies, University of Southern Denmark (SDU), Centrovice (the local agricultural trade organisation), and Udvikling Fyn (a regional business development company). In total, 36 students from SDU have made their Christian Tønnesen, Project manager and Head of the diploma work under the auspices of the energy plan Settlement & Business Department, Faaborg-Midtfyn thus creating a win-win satiation for students, Municipality, underlines: “The ambition for Funen university, local businesses, and municipalities. Energy Plan is to create a platform for the energy Exporting biomass fuel instead of electricity stakeholders, so that they from a common point of view can approach a joint planning for the future The energy plan presents a number of energy with a focus on innovation and optimal recommendations linked to particularly challenging solutions.” energy areas, where robust and long-term navigation Students contributing to real life energy plans is crucial. -

Kommunenavn: Svendborg Kommuners Indberetning På
Kommunenavn: Svendborg Kommuners indberetning på godsområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Billetindtægter er ekskl. ordinært § 21 a tilskud, vareafgift til havnene samt moms Beregning af mindreindtægter Billetindtægter i Billetindtægter i perioden marts- perioden marts- september i 2019 (i september i 2020 (i Kommune Rute 1.000 kr.) 1.000 kr.) Assens Baagø-Assens Fanø Fanø-Esbjerg Faaborg-Midtfyn Bjørnø-Faaborg Faaborg-Midtfyn Lyø-Avernakø-Faaborg Haderslev Aarø-Aarøsund Hedensted Hjarnø-Snaptun Holbæk Orø-Holbæk Holbæk Orø- Hammer bakker Horsens Endelave-Snaptun Kalundborg Havnsø-Sejerø Kalundborg Havnsø-Nekselø Langeland Strynø-Rudkøbing Lolland Fejø-Kragenæs Lolland Femø-Kragenæs Lolland Askø-Bandholm Læsø Læsø-Frederikshavn Norddjurs Anholt-Grenaa Odder Tunø-Hou Samsø Hou-Samsø Skive Fur-Branden Slagelse Agersø-Stigsnæs Slagelse Omø-Stigsnæs Struer Venø-Kleppen Svendborg Hjortø-Svendborg 1 3 Svendborg Skarø-drejø-Svendborg 139 111 Ærø Birkholm-Marstal Ærø Ærøskøbing-Svendborg Ærø Søby-Faaborg Ærø Søby-Fynshav Ærø Marstal-Rudkøbing Aabenraa Barsø-Barsø-Landing Aalborg Egholm-Aalborg Kommuners indberetning på godsområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Beregning af mindreindtægter Samlede mindreindtægte r i 2020 (i 1.000 kr.) 2 -28 Kommunenavn: Svendborg Kommuners indberetning på passagerområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Billetindtægt er ekskl. ordinært § 21 b tilskud og moms Beregning af mindreindtægter Billetindtægter -

Dueodde Natura 2000-Område Nr
Natura 2000 plan 2016-21 Kolofon Titel: År: Natura 2000-plan 2016-2021 2016 Dueodde Natura 2000-område nr. 188 ISBN nr. Habitatområde H164 978-87-7091-882-4 Emneord: Dato: Habitatdirektivet, fuglebeskyttelsesdirektivet, Februar 2016 Miljømålsloven, Natura 2000-plan. Forsidefoto: Udgiver: Bornholms Regionskommune Miljø- og Fødevareministeriet, Naturstyrelsen Resume: Ansvarlig institution: Natura 2000-plan for Dueodde (nr. 188). Natura Naturstyrelsen 2000-planen skal sikre naturtilstanden for Haraldsgade 53 områdets udpegede arter og naturtyper og 2100 København Ø bidrage til opnåelse af gunstig bevaringsstatus. www.naturstyrelsen.dk Områdets udpegede naturtyper og arter beskrives. Der er foretaget tilstandsvurdering af Copyright: levesteder og naturtyper, og der fastlægges Naturstyrelsen, Miljø- og Fødevareministeriet målsætninger og indsatser for naturtyperne og arterne. Sprog: Dansk Må citeres med kildeangivelse 2 Natura 2000 plan 2016-21 Indhold 1. Natura 2000-planlægning .................................................................................. 5 1.1 Natura 2000-planen er bindende for myndighederne ......................................................... 6 1.2 Naturtilstand og gunstig bevaringsstatus ..............................................................................7 2. Områdebeskrivelse ............................................................................................. 8 2.1 Områdets udpegningsgrundlag ............................................................................................. 9 2.2 -

Island Living on Bornholm
To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Island living on Bornholm © Semko Balcerski To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Land of many islands In Denmark, we look for a touch of magic in the ordinary, and we know that travel is more than ticking sights off a list. It’s about finding the wonder in the things you see and the places you go. One of the wonders, that we at VisitDenmark are particularly proud of, is our nature. Denmark has hundreds of islands, each with their own unique appeal. The island of Bornholm in the Baltic sea is known for its soft adventures, sustainability, gastronomy and impressive nature. s. 2 © Stefan Asp To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Denmark and its regions Geography Travel distances Aalborg • The smallest of the Scandinavian • Copenhagen to Odense: Bornholm countries Under 2 hours by car • The southernmost of the • Odense to Aarhus: Under 2 Scandinavian countries hours by car • Only has a physical border with • Aarhus to Aalborg: Under 2 Germany hours by car • Denmark’s regions are: North, Mid, Jutland West and South Jutland, Funen, Aarhus Zealand, and North Zealand and Copenhagen Billund Facts Copenhagen • Video Introduction • Denmark’s currency is the Danish Kroner Odense • Tipping is not required Zealand • Most Danes speak fluent English Funen • Denmark is of the happiest countries in the world and Copenhagen is one of the world’s most liveable cities • Denmark is home of ‘Hygge’, New Nordic Cuisine, and LEGO® • Denmark is easily combined with other Nordic countries • Denmark is a safe country • Denmark is perfect for all types of travelers (family, romantic, nature, bicyclist dream, history/Vikings/Royalty) • Denmark has a population of 5.7 million people s. -

Miljøvurdering Af Fællesaftalen for Skagen
FÆLLESAFTALE OM KYSTBESKYT- TELSE PÅ STRÆKNINGEN SKA- GEN MILJØVURDERING Miljøvurdering af fællesaftalen KOLOFON Titel: Miljøvurdering af fællesaftalen for kystbeskyttelse Skagen Udgiver: Kystdirektoratet, Kystbeskyttelse - Drift og anlæg Forfatter: Rambøll År: 2020 Rambøll Hannemanns Allé 53 DK-2300 København S T +45 5161 1000 F +45 5161 1001 www.ramboll.dk | 1/99 MILJØVURDERING AF FÆLLESAFTALEN KYSTBESKYTTELSE VED SKAGEN INDHOLD 1. INDLEDNING 3 2. IKKE-TEKNISK RESUMÉ 6 3. BESKRIVELSE AF FÆLLESAFTALENS INDHOLD 9 4. MILJØVURDERINGENS INDHOLD OG METODE 12 5. FORHOLD TIL ANDEN PLANLÆGNING 14 6. ALTERNATIVER 18 7. LANDSKAB 20 8. KYSTDYNAMIK, STRØMNING OG SEDIMENTATION 32 9. VAND 38 10. LUFT 42 11. KLIMA 45 12. JORD 49 13. MARIN BUNDFAUNA 52 14. FISK 58 15. HAVPATTEDYR, HAVFUGLE, BESKYTTEDE MARINE OMRÅDER OG BILAG IV- ARTER 62 16. NATUR PÅ LAND 70 17. KULTURARV OG HISTORISKE INTERESSER 77 18. MATERIELLE GODER 82 19. TURISME OG REKREATION 85 20. BEFOLKNING OG MENNESKERS SUNDHED 89 21. KUMULATIVE EFFEKTER 94 22. AFVÆRGETILTAG 97 23. SAMMENFATTENDE VURDERING 98 24. OVERVÅGNING 99 | 2/99 MILJØVURDERING AF FÆLLESAFTALEN KYSTBESKYTTELSE VED SKAGEN 1. INDLEDNING 1.1 Baggrund for fællesaftalen Siden 1982 har kystbeskyttelsesindsatsen på den 4,4 meter lange strækning ved Skagen været fastlagt på grundlag af et- og femårige fællesaftaler mellem staten, daværende Nordjyllands Amt og Frederikshavn Kommune. Fællesaftalen mellem Frederikshavn Kommune og staten, som gæl- der i perioden 2020-2024, er en forlængelse af den forrige femårige aftale for perioden 2014- 2018(19). Den nye fællesaftale mellem Frederikshavn Kommune og staten omfatter perioden 2020-24 og består af en økonomisk ramme, hvor det overordnede formål er, at kysten så vidt muligt bevares som den er i dag. -

Development and Testing of Tools for Intercalibration of Phytoplankton, Macrovegetation and Benthic Fauna in Danish Coastal Areas
DEVELOPMENT AND TESTING OF TOOLS FOR INTERCALIBRATION OF PHYTOPLANKTON, MACROVEGETATION AND BENTHIC FAUNA IN DANISH COASTAL AREAS Scientifi c Report from DCE – Danish Centre for Environment and Energy No. 93 2014 AARHUS AU UNIVERSITY DCE – DANISH CENTRE FOR ENVIRONMENT AND ENERGY [Blank page] DEVELOPMENT AND TESTING OF TOOLS FOR INTERCALIBRATION OF PHYTOPLANKTON, MACROVEGETATION AND BENTHIC FAUNA IN DANISH COASTAL AREAS Scientifi c Report from DCE – Danish Centre for Environment and EnergyNo. 93 2014 Jacob Carstensen Dorte Krause-Jensen Alf Josefson Aarhus University, Department of Bioscience AARHUS AU UNIVERSITY DCE – DANISH CENTRE FOR ENVIRONMENT AND ENERGY Data sheet Series title and no.: Scientific Report from DCE – Danish Centre for Environment and Energy No. 93 Title: Development and testing of tools for intercalibration of phytoplankton, macrovegetation and benthic fauna in Danish coastal areas Authors: Jacob Carstensen, Dorte Krause-Jensen, Alf Josefson Institution: Aarhus University, Department of Bioscience Publisher: Aarhus University, DCE – Danish Centre for Environment and Energy © URL: http://dce.au.dk/en Year of publication: March 2014 Editing completed: February 2014 Referees: Peter Henriksen, Department of Bioscience, Aarhus University Financial support: Danish Nature Agency (NST) Please cite as: Carstensen, J., Krause-Jensen, D., Josefson, A. 2014. Development and testing of tools for intercalibration of phytoplankton, macrovegetation and benthic fauna in Danish coastal areas. Aarhus University, DCE – Danish Centre for Environment and Energy, 85 pp. Scientific Report from DCE – Danish Centre for Environment and Energy No. 93. http://dce2.au.dk/pub/SR93.pdf Reproduction permitted provided the source is explicitly acknowledged Abstract: This report contributes to the development of indicators and assessment tools for ecological status classification according to the European Water Framework Directive as well as the intercalibration of the phytoplankton biomass indicator with Sweden and Germany. -

Coastal Living in Denmark
To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Coastal living in Denmark © Daniel Overbeck - VisitNordsjælland To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. The land of endless beaches In Denmark, we look for a touch of magic in the ordinary, and we know that travel is more than ticking sights off a list. It’s about finding wonder in the things you see and the places you go. One of the wonders that we at VisitDenmark are particularly proud of is our nature. Denmark has wonderful beaches open to everyone, and nowhere in the nation are you ever more than 50km from the coast. s. 2 © Jill Christina Hansen To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Denmark and its regions Geography Travel distances Aalborg • The smallest of the Scandinavian • Copenhagen to Odense: Bornholm countries Under 2 hours by car • The southernmost of the • Odense to Aarhus: Under 2 Scandinavian countries hours by car • Only has a physical border with • Aarhus to Aalborg: Under 2 Germany hours by car • Denmark’s regions are: North, Mid, Jutland West and South Jutland, Funen, Aarhus Zealand, and North Zealand and Copenhagen Billund Facts Copenhagen • Video Introduction • Denmark’s currency is the Danish Kroner Odense • Tipping is not required Zealand • Most Danes speak fluent English Funen • Denmark is of the happiest countries in the world and Copenhagen is one of the world’s most liveable cities • Denmark is home of ‘Hygge’, New Nordic Cuisine, and LEGO® • Denmark is easily combined with other Nordic countries • Denmark is a safe country • Denmark is perfect for all types of travelers (family, romantic, nature, bicyclist dream, history/Vikings/Royalty) • Denmark has a population of 5.7 million people s. -

Invasive Ctenophore Mnemiopsis Leidyi Widely Distributed in Danish Waters
Aquatic Invasions (2007) Volume 2, Issue 4: 455-460 DOI 10.3391/ai.2007.2.4.19 © 2007 The Author(s) Journal compilation © 2007 REABIC (http://www.reabic.net) This is an Open Access article Special issue “Alien species in European coastal waters” Geoff Boxshall, Ferdinando Boero and Sergej Olenin (Guest Editors) Short communication Invasive ctenophore Mnemiopsis leidyi widely distributed in Danish waters Ole Secher Tendal1*, Kathe R. Jensen1,2 and Hans Ulrik Riisgård3 1Zoological Museum, SNM, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark E-mail: [email protected] 2Agency for Spatial and Environmental Planning, Haraldsgade 53, DK-2100 Copenhagen Ø, Denmark E-mail: [email protected] 3Marine Biological Research Centre, University of Southern Denmark, Hindsholmvej 11, DK- 5300 Kerteminde, Denmark E-mail: [email protected] *Corresponding author Received 27 September 2007; accepted in revised form 22 October 2007 Abstract Blooms of Mnemiopsis leidyi observed along the coast of The Netherlands in late 2006 have made the spreading of this invasive ctenophore to neighboring waters a topic of major concern. Here we report on recent occurrences of M. leidyi in Danish waters, observed partly by ourselves and other biologists, partly by beach guests, boat owners and amateur divers. The earliest record of M. leidyi is from August 2005 and the early summer of 2006, and in 2007, the earliest records are from February and March, from the northern Little Belt and Kerteminde Bay. In the period April to June, the density of M. leidyi remained very low in the Great Belt, but numerous reports indicate that the ctenophore in July to September was widely distributed in all inner Danish waters, and "mass occurrences" have been reported from certain areas.