Neuropeptidases, Stress, and Memory—A Promising Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzymatic Encoding Methods for Efficient Synthesis Of
(19) TZZ__T (11) EP 1 957 644 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/10 (2006.01) C12Q 1/68 (2006.01) 01.12.2010 Bulletin 2010/48 C40B 40/06 (2006.01) C40B 50/06 (2006.01) (21) Application number: 06818144.5 (86) International application number: PCT/DK2006/000685 (22) Date of filing: 01.12.2006 (87) International publication number: WO 2007/062664 (07.06.2007 Gazette 2007/23) (54) ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES ENZYMVERMITTELNDE KODIERUNGSMETHODEN FÜR EINE EFFIZIENTE SYNTHESE VON GROSSEN BIBLIOTHEKEN PROCEDES DE CODAGE ENZYMATIQUE DESTINES A LA SYNTHESE EFFICACE DE BIBLIOTHEQUES IMPORTANTES (84) Designated Contracting States: • GOLDBECH, Anne AT BE BG CH CY CZ DE DK EE ES FI FR GB GR DK-2200 Copenhagen N (DK) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • DE LEON, Daen SK TR DK-2300 Copenhagen S (DK) Designated Extension States: • KALDOR, Ditte Kievsmose AL BA HR MK RS DK-2880 Bagsvaerd (DK) • SLØK, Frank Abilgaard (30) Priority: 01.12.2005 DK 200501704 DK-3450 Allerød (DK) 02.12.2005 US 741490 P • HUSEMOEN, Birgitte Nystrup DK-2500 Valby (DK) (43) Date of publication of application: • DOLBERG, Johannes 20.08.2008 Bulletin 2008/34 DK-1674 Copenhagen V (DK) • JENSEN, Kim Birkebæk (73) Proprietor: Nuevolution A/S DK-2610 Rødovre (DK) 2100 Copenhagen 0 (DK) • PETERSEN, Lene DK-2100 Copenhagen Ø (DK) (72) Inventors: • NØRREGAARD-MADSEN, Mads • FRANCH, Thomas DK-3460 Birkerød (DK) DK-3070 Snekkersten (DK) • GODSKESEN, -

PROTEOMIC ANALYSIS of HUMAN URINARY EXOSOMES. Patricia
ABSTRACT Title of Document: PROTEOMIC ANALYSIS OF HUMAN URINARY EXOSOMES. Patricia Amalia Gonzales Mancilla, Ph.D., 2009 Directed By: Associate Professor Nam Sun Wang, Department of Chemical and Biomolecular Engineering Exosomes originate as the internal vesicles of multivesicular bodies (MVBs) in cells. These small vesicles (40-100 nm) have been shown to be secreted by most cell types throughout the body. In the kidney, urinary exosomes are released to the urine by fusion of the outer membrane of the MVBs with the apical plasma membrane of renal tubular epithelia. Exosomes contain apical membrane and cytosolic proteins and can be isolated using differential centrifugation. The analysis of urinary exosomes provides a non- invasive means of acquiring information about the physiological or pathophysiological state of renal cells. The overall objective of this research was to develop methods and knowledge infrastructure for urinary proteomics. We proposed to conduct a proteomic analysis of human urinary exosomes. The first objective was to profile the proteome of human urinary exosomes using liquid chromatography-tandem spectrometry (LC- MS/MS) and specialized software for identification of peptide sequences from fragmentation spectra. We unambiguously identified 1132 proteins. In addition, the phosphoproteome of human urinary exosomes was profiled using the neutral loss scanning acquisition mode of LC-MS/MS. The phosphoproteomic profiling identified 19 phosphorylation sites corresponding to 14 phosphoproteins. The second objective was to analyze urinary exosomes samples isolated from patients with genetic mutations. Polyclonal antibodies were generated to recognize epitopes on the gene products of these genetic mutations, NKCC2 and MRP4. The potential usefulness of urinary exosome analysis was demonstrated using the well-defined renal tubulopathy, Bartter syndrome type I and using the single nucleotide polymorphism in the ABCC4 gene. -

Cloud-Clone 16-17
Cloud-Clone - 2016-17 Catalog Description Pack Size Supplier Rupee(RS) ACB028Hu CLIA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 74750 AEA044Hu ELISA Kit for Anti-Growth Hormone Antibody (Anti-GHAb) 96T Cloud-Clone 74750 AEA255Hu ELISA Kit for Anti-Apolipoprotein Antibodies (AAHA) 96T Cloud-Clone 74750 AEA417Hu ELISA Kit for Anti-Proteolipid Protein 1, Myelin Antibody (Anti-PLP1) 96T Cloud-Clone 74750 AEA421Hu ELISA Kit for Anti-Myelin Oligodendrocyte Glycoprotein Antibody (Anti- 96T Cloud-Clone 74750 MOG) AEA465Hu ELISA Kit for Anti-Sperm Antibody (AsAb) 96T Cloud-Clone 74750 AEA539Hu ELISA Kit for Anti-Myelin Basic Protein Antibody (Anti-MBP) 96T Cloud-Clone 71250 AEA546Hu ELISA Kit for Anti-IgA Antibody 96T Cloud-Clone 71250 AEA601Hu ELISA Kit for Anti-Myeloperoxidase Antibody (Anti-MPO) 96T Cloud-Clone 71250 AEA747Hu ELISA Kit for Anti-Complement 1q Antibody (Anti-C1q) 96T Cloud-Clone 74750 AEA821Hu ELISA Kit for Anti-C Reactive Protein Antibody (Anti-CRP) 96T Cloud-Clone 74750 AEA895Hu ELISA Kit for Anti-Insulin Receptor Antibody (AIRA) 96T Cloud-Clone 74750 AEB028Hu ELISA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 71250 AEB264Hu ELISA Kit for Insulin Autoantibody (IAA) 96T Cloud-Clone 74750 AEB480Hu ELISA Kit for Anti-Mannose Binding Lectin Antibody (Anti-MBL) 96T Cloud-Clone 88575 AED245Hu ELISA Kit for Anti-Glutamic Acid Decarboxylase Antibodies (Anti-GAD) 96T Cloud-Clone 71250 AEK505Hu ELISA Kit for Anti-Heparin/Platelet Factor 4 Antibodies (Anti-HPF4) 96T Cloud-Clone 71250 CCA005Hu CLIA Kit for Angiotensin II -

Formylpeptide Receptor N Antagonist at The
The Endogenous Opioid Spinorphin Blocks fMet-Leu-Phe-Induced Neutrophil Chemotaxis by Acting as a Specific Antagonist at the N-Formylpeptide Receptor This information is current as Subtype FPR of October 1, 2021. Thomas S. Liang, Ji-Liang Gao, Omid Fatemi, Mark Lavigne, Thomas L. Leto and Philip M. Murphy J Immunol 2001; 167:6609-6614; ; doi: 10.4049/jimmunol.167.11.6609 Downloaded from http://www.jimmunol.org/content/167/11/6609 References This article cites 48 articles, 13 of which you can access for free at: http://www.jimmunol.org/content/167/11/6609.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Endogenous Opioid Spinorphin Blocks fMet-Leu-Phe-Induced Neutrophil Chemotaxis by Acting as a Specific Antagonist at the N-Formylpeptide Receptor Subtype FPR Thomas S. -

Characterization of Aminopeptidase in the Free-Living Nematode Panagrellus Redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E
Journal of Nematology 39(2):153–160. 2007. © The Society of Nematologists 2007. Characterization of Aminopeptidase in the Free-living Nematode Panagrellus redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E. P. Masler Abstract: Aminopeptidase was detected in homogenates of the free-living nematode Panagrellus redivivus with the aminoacyl substrate L-alanine-4-nitroanilide. Subcellular distribution of activity was 80% soluble and 20% membrane-associated. Aminopep- tidases in the two fractions differed in affinity for Ala-4-NA, with Km’s of 0.65 mM (soluble) and 2.90 mM (membrane). Specific activities (units/mg) at pH 7.8, 27°C were 9.10 (soluble) and 14.30 (membrane). Each enzyme was competitively inhibited by amastatin (90% at 100 µM inhibitor, IC50 = 3.7 µM) and inhibited by puromycin (30% at 500 µM) and 1,10-phenanthroline (IC50’s:; 148 µM, soluble; 89 µM, membrane). Activity was restored by Zn++, with maximum recoveries of 50% (soluble) and 90% (mem- ∼ brane), each at 23 µM ZnCl2. Estimated molecular masses for each were 150 kDa. FMRFamide-like neuropeptides behaved as competitive inhibitors. Modification of the N-terminal F of FMRFamide weakened inhibition by 95%, suggesting that the N-terminus is essential for binding to the enzyme. Two nematode FMRFamides, APKPFIRFa and RNKFEFIRFa, were the most potent tested. This is the first biochemical characterization of aminopeptidase in a free-living nematode other than Caenorhabditis elegans and demon- strates the high selectivity of the P. redivivus enzymes for neuropeptide substrates. Key words: FMRFamide-like peptide, inhibitor; membrane, metallopeptidase, neuropeptide, protease Nematodes, like other eukaryotic organisms, depend reproduction (Day and Maule, 1999; Maule et al., 2002; upon proteolytic enzymes for the regulation of essential Rogers et al., 2003). -

Code Lists Page 1
Code lists Page 1 Code lists AESEV Page 2 AESEV Codelist Name: Severity/Intensity Scale for Adverse Events Description: A scale that defines the degree or state of disease existing in a patient as a result of the occurrence of an adverse event. (NCI) C41338,1; Grade 1 C41339,2; Grade 2 C41340,3; Grade 3 AGEU Page 3 AGEU Codelist Name: Age Unit Description: Those units of time that are routinely used to express the age of a subject. C25301,Days C25529,Hours; h; hr C29846,Month C29844,Week C29848,Year CMDOSFRM Page 4 CMDOSFRM Codelist Name: Concomitant Medication Dose Form Description: A terminology subset of the CDISC SDTM Pharmaceutical Dosage Form codelist created for CDASH Concomitant Medication Dose Form codelist. (NCI) C42887,AEROSOL; aer C25158,CAPSULE; cap C28944,CREAM; C42933,GAS; C42934,GEL; C42966,OINTMENT; oint C42968,PATCH; C42972,POWDER; C42989,SPRAY; C42993,SUPPOSITORY; supp C42994,SUSPENSION; susp C42998,TABLET; tab CMDOSFRQ Page 5 CMDOSFRQ Codelist Name: Concomitant Medication Dosing Frequency per Interval Description: A terminology subset of the CDISC SDTM Frequency codelist created for CDASH Concomitant Medication Dosing Frequency per Interval codelist. (NCI) C64496,BID; BD; Twice per day C64499,PRN; As needed C25473,QD; Daily C64530,QID; 4 times per day C64498,QM; Every Month; Per Month C64525,QOD; Every other day C64527,TID; 3 times per day C17998,UNKNOWN; U; UNK; Unknown CMDOSU Page 6 CMDOSU Codelist Name: Concomitant Medication Dose Units Description: A terminology subset of the CDISC SDTM Unit codelist created for CDASH Concomitant Medication Dose Units codelist. (NCI) C48480,CAPSULE; Capsule Dosing Unit; cap C48155,g; Gram C48579,IU; IE; International Unit C28253,mg; Milligram C28254,mL; Milliliter; cm3 C65060,PUFF; Puff Dosing Unit C48542,TABLET; Tablet Dosing Unit; tab C48152,ug; Microgram; mcg CMROUTE Page 7 CMROUTE Codelist Name: Concomitant Medication Route of Administration Description: A terminology subset of the CDISC SDTM Route codelist created for CDASH Concomitant Medication Route of Administration codelist. -

Opioid Peptides 49 Ryszard Przewlocki
Opioid Peptides 49 Ryszard Przewlocki Abbreviations ACTH Adrenocorticotropic hormone CCK Cholecystokinin CPA Conditioned place aversion CPP Conditioned place preference CRE cAMP response element CREB cAMP response element binding CRF Corticotrophin-releasing factor CSF Cerebrospinal fluid CTAP D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (m-opioid receptor antagonist) DA Dopamine DOP d-opioid peptide EOPs Endogenous opioid peptides ERK Extracellular signal-regulated kinase FSH Follicle-stimulating hormone GnRH Gonadotrophin-releasing hormone HPA axis Hypothalamo-pituitary-adrenal axis KO Knockout KOP k-opioid peptide LH Luteinizing hormone MAPK Mitogen-activated protein kinase MOP m-opioid peptide NOP Nociceptin opioid peptide NTS Nucleus tractus solitarii PAG Periaqueductal gray R. Przewlocki Department of Molecular Neuropharmacology, Institute of Pharmacology, PAS, Krakow, Poland Department of Neurobiology and Neuropsychology, Jagiellonian University, Krakow, Poland e-mail: [email protected] D.W. Pfaff (ed.), Neuroscience in the 21st Century, 1525 DOI 10.1007/978-1-4614-1997-6_54, # Springer Science+Business Media, LLC 2013 1526 R. Przewlocki PDYN Prodynorphin PENK Proenkephalin PNOC Pronociceptin POMC Proopiomelanocortin PTSD Posttraumatic stress disorder PVN Paraventricular nucleus SIA Stress-induced analgesia VTA Ventral tegmental area Brief History of Opioid Peptides and Their Receptors Man has used opium extract from poppy seeds for centuries for both pain relief and recreation. At the beginning of the nineteenth century, Serturmer first isolated the active ingredient of opium and named it morphine after Morpheus, the Greek god of dreams. Fifty years later, morphine was introduced for the treatment of postoper- ative and chronic pain. Like opium, however, morphine was found to be an addictive drug. -

Opioids, Neutral Endopeptidase, Its Inhibitors and Cancer: Is There a Relationship Among Them?
Arch. Immunol. Ther. Exp. DOI 10.1007/s00005-014-0311-0 REVIEW ARTICLE Opioids, Neutral Endopeptidase, its Inhibitors and Cancer: Is There a Relationship among them? Magdalena Mizerska-Dudka • Martyna Kandefer-Szerszen´ Received: 11 March 2014 / Accepted: 18 June 2014 Ó The Author(s) 2014. This article is published with open access at Springerlink.com Abstract The role of endogenous animal opioids in the CKI Cyclin dependent inhibitory kinases biology of cancer is widely recognized but poorly under- ECM Extracellular matrix stood. This is, among others, because of the short half-life FAK Focal adhesion kinase of these peptides, which are quickly inactivated by endo- GPI-complex Glycosyl phosphatidyl inositol complex peptidases, e.g., neutral endopeptidase (NEP, CD10). It has MAP kinases Mitogen-activated protein kinases been established that NEP is engaged in the modulation of mRNA Messenger RNA the tumor microenvironment, among others that of colon NEP Neutral endopeptidase cancer, by exerting influence on cell growth factors, the NK Natural killer cells extracellular matrix and other biologically active sub- OGF Opioid growth factor stances. Although there are some discrepancies among the OGFr Opioid growth factor receptor findings on the role of both opioids and NEP in cancer PROL1 Proline rich, lacrimal 1 development, authors agree that their role seems to depend PTEN Phosphatase and tensin homolog deleted on the origin, stage and grade of tumor, and even on the on chromosome Ten method of examination. Moreover, recently, natural SGP-T Submandibular gland peptide-T inhibitors of NEP, such as sialorphin, opiorphin and spin- SMR1 Submandibular rat1 protein orphin have been detected. -
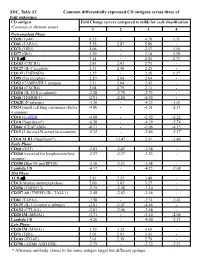
SDC, Table S1. Common Differentially Expressed CD Antigens
SDC, Table S1. Common differentially expressed CD antigens across three of four outcomes CD antigen Fold Change (severe compared to mild) for each classification (Common or alternate name) 1 2 3 4 Pretransplant Phase CD28 (Tp44) 6.32 - 4.79 3.71 CD81 (TAPA1) 5.58 2.87 5.86 - CD71 (TfR1) 4.06 - 2.57 3.16 CD77 (Gb3) 3.29 - 3.18 3.29 TCR ab 3.14 - 2.01 2.73 CD183 (CXCR3) 2.43 2.93 2.71 - CD127 (IL-7 receptor) 2.31 2.48 2.25 - CD137 (TNFRSF9) 2.27 - 2.35 2.27 CD95 (Fas receptor) 2.20 2.04 2.64 - CD52 (CAMPATH-1 antigen) 2.11 2.04 2.43 - CD184 (CXCR4) 2.04 2.79 2.11 - CD210 (IL-10 R a subunit) -2.28 -2.79 -2.73 - CD40 (TNFRSF5) -2.91 -2.20 -4.92 - CD62E (E-selectin) -3.36 - -4.23 -3.01 CD24 (small cell lung carcinoma cluster -4.00 - -3.51 -2.33 4 antigen) CD16 (FcγRIII) -4.08 - -2.92 -2.22 CD10 (Neprilysin*) -6.28 - -4.29 -5.74 CD66c (CEACAM6) -8.51 - -5.06 -6.15 CD15 (3-fucosyl-N-acetyl-lactosamine) -9.32 - -5.86 -5.17 CD10 ALB1 (Neprilysin*) - 12.47 2.51 -3.46 Early Phase CD60 (GD3) -2.03 -2.43 -2.58 - CD260 (reserved for lymphotoxin beta -2.57 -2.27 -3.23 - receptor) CD180 (Bgp-95 and RP105) -3.10 -2.33 -3.48 - Lambda 1/8 -4.17 - -4.23 -2.68 Mid Phase TCR ab 2.81 2.22 3.48 - CD13(Alanine aminopeptidase) 2.00 3.03 3.27 - CD256 (TNFSF13) -2.19 -2.48 -3.10 - CD257 AS (TNFSF13B / TALL1) -2.48 -2.62 -3.46 - CD81 (TAPA1) -2.58 - -2.31 -2.43 CD125 (IL-5 receptor a subunit) -2.81 -3.07 -4.66 - CD152 (CTLA-4) -2.81 -2.06 -3.48 - CD20 IM (MS4A1) -3.71 - -3.10 -2.04 Lambda 1/8 -4.26 - -4.00 -2.13 Late Phase CD20 IM (MS4A1) 3.39 3.32 4.69 - CD71 (TfR1) 2.03 2.93 3.01 - CD103 (ITGAE) 2.03 2.39 2.79 - CD79b (AGM6 AND IGB) -2.79 - -2.32 -2.23 * Alternate antibody clones to the same antigen target but different epitope. -

Aminopeptidases in Cardiovascular and Renal Function. Role As Predictive Renal Injury Biomarkers
International Journal of Molecular Sciences Review Aminopeptidases in Cardiovascular and Renal Function. Role as Predictive Renal Injury Biomarkers Félix Vargas 1, Rosemary Wangesteen 2, Isabel Rodríguez-Gómez 1 and Joaquín García-Estañ 3,* 1 Depto. Fisiologia, Fac. Medicina, Universidad de Granada, 18071 Granada, Spain; [email protected] (F.V.); [email protected] (I.R.-G.) 2 Depto. Ciencias de la salud, Universidad de Jaén, 23071 Jaén, Spain; [email protected] 3 Depto. Fisiologia, Fac. Medicina, IMIB, Universidad de Murcia, 30120 Murcia, Spain * Correspondence: [email protected] Received: 23 June 2020; Accepted: 3 August 2020; Published: 5 August 2020 Abstract: Aminopeptidases (APs) are metalloenzymes that hydrolyze peptides and polypeptides by scission of the N-terminus amino acid and that also participate in the intracellular final digestion of proteins. APs play an important role in protein maturation, signal transduction, and cell-cycle control, among other processes. These enzymes are especially relevant in the control of cardiovascular and renal functions. APs participate in the regulation of the systemic and local renin–angiotensin system and also modulate the activity of neuropeptides, kinins, immunomodulatory peptides, and cytokines, even contributing to cholesterol uptake and angiogenesis. This review focuses on the role of four key APs, aspartyl-, alanyl-, glutamyl-, and leucyl-cystinyl-aminopeptidases, in the control of blood pressure (BP) and renal function and on their association with different cardiovascular and renal diseases. In this context, the effects of AP inhibitors are analyzed as therapeutic tools for BP control and renal diseases. Their role as urinary biomarkers of renal injury is also explored. The enzymatic activities of urinary APs, which act as hydrolyzing peptides on the luminal surface of the renal tubule, have emerged as early predictive renal injury biomarkers in both acute and chronic renal nephropathies, including those induced by nephrotoxic agents, obesity, hypertension, or diabetes. -

Fragmentación Del Adn Espermático Y Su Relación Con El Metabolismo Peptídico
Facultad de Medicina y Enfermería Medikuntza eta Erizaintza Fakultatea Departamento de Fisiología Fisiologia Saila TESIS DOCTORAL FRAGMENTACIÓN DEL ADN ESPERMÁTICO Y SU RELACIÓN CON EL METABOLISMO PEPTÍDICO MEMORIA PRESENTADA POR: Mª Victoria Aparicio Prieto DIRIGIDA POR LOS DOCTORES: Luis Casis Saenz Nerea Subirán Ciudad 2018 TESIS DOCTORAL Facultad de Medicina y Enfermería Medikuntza eta Erizaintza Fakultatea Departamento de Fisiología Fisiologia Saila TESIS DOCTORAL FRAGMENTACIÓN DEL ADN ESPERMÁTICO Y SU RELACIÓN CON EL METABOLISMO PEPTÍDICO MEMORIA PRESENTADA POR: Mª Victoria Aparicio Prieto DIRIGIDA POR LOS DOCTORES: LUIS CASIS SAENZ NEREA SUBIRÁN CIUDAD 2018 Mª Victoria Aparicio Prieto 1 (cc)2018 Mª VICTORIA APARICIO PRIETO (cc by-nc 4.0) TESIS DOCTORAL Mª Victoria Aparicio Prieto 12 TESIS DOCTORAL AGRADECIMIENTOS No sólo los conocimientos científicos son importantes, sino que es tan importante tener al lado un gran equipo humano, generoso y entusiasta. Por lo que quiero decir “GRACIAS” a todas estas personas que han compartido conmigo este proyecto. En primer lugar, me gustaría agradecer este estudio al profesor Dr. Luis Casis, mi director, una gran persona no solo en el ámbito profesional y académico sino también en la parte humana. Gracias a él he podido realizar esta investigación. Ha sido mi compañero en los momentos de bloqueo y un buen tutor cuando necesitaba orientación y apoyo científico. Su amistad, ayuda incondicional más allá de lo que su papel como director exige, interés, actitud siempre positiva y dispuesta para que este proyecto avanzara. Mi agradecimiento a la Dra. Olga Ramón que fue mi codirectora de este proyecto hasta su fallecimiento. Mujer emblemática, luchadora incansable, que me dio la oportunidad de no declinar cuando las cosas se veían difíciles, y que a estas alturas de mi vida me animó a llevar a cabo este proyecto. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0081648A1 Afeyan Et Al
US 2004.008 1648A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0081648A1 Afeyan et al. (43) Pub. Date: Apr. 29, 2004 (54) ADZYMES AND USES THEREOF Publication Classification (76) Inventors: Noubar B. Afeyan, Lexington, MA (51) Int. Cl." ............................. A61K 38/48; C12N 9/64 (US); Frank D. Lee, Chestnut Hill, MA (52) U.S. Cl. ......................................... 424/94.63; 435/226 (US); Gordon G. Wong, Brookline, MA (US); Ruchira Das Gupta, Auburndale, MA (US); Brian Baynes, (57) ABSTRACT Somerville, MA (US) Disclosed is a family of novel protein constructs, useful as Correspondence Address: drugs and for other purposes, termed “adzymes, comprising ROPES & GRAY LLP an address moiety and a catalytic domain. In Some types of disclosed adzymes, the address binds with a binding site on ONE INTERNATIONAL PLACE or in functional proximity to a targeted biomolecule, e.g., an BOSTON, MA 02110-2624 (US) extracellular targeted biomolecule, and is disposed adjacent (21) Appl. No.: 10/650,592 the catalytic domain So that its affinity Serves to confer a new Specificity to the catalytic domain by increasing the effective (22) Filed: Aug. 27, 2003 local concentration of the target in the vicinity of the catalytic domain. The present invention also provides phar Related U.S. Application Data maceutical compositions comprising these adzymes, meth ods of making adzymes, DNA's encoding adzymes or parts (60) Provisional application No. 60/406,517, filed on Aug. thereof, and methods of using adzymes, Such as for treating 27, 2002. Provisional application No. 60/423,754, human Subjects Suffering from a disease, Such as a disease filed on Nov.