Defining Physiological Differences Between Gilts Divergently Selected for Residual Feed Intake Amanda Jean Harris Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enzymatic Encoding Methods for Efficient Synthesis Of
(19) TZZ__T (11) EP 1 957 644 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12N 15/10 (2006.01) C12Q 1/68 (2006.01) 01.12.2010 Bulletin 2010/48 C40B 40/06 (2006.01) C40B 50/06 (2006.01) (21) Application number: 06818144.5 (86) International application number: PCT/DK2006/000685 (22) Date of filing: 01.12.2006 (87) International publication number: WO 2007/062664 (07.06.2007 Gazette 2007/23) (54) ENZYMATIC ENCODING METHODS FOR EFFICIENT SYNTHESIS OF LARGE LIBRARIES ENZYMVERMITTELNDE KODIERUNGSMETHODEN FÜR EINE EFFIZIENTE SYNTHESE VON GROSSEN BIBLIOTHEKEN PROCEDES DE CODAGE ENZYMATIQUE DESTINES A LA SYNTHESE EFFICACE DE BIBLIOTHEQUES IMPORTANTES (84) Designated Contracting States: • GOLDBECH, Anne AT BE BG CH CY CZ DE DK EE ES FI FR GB GR DK-2200 Copenhagen N (DK) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • DE LEON, Daen SK TR DK-2300 Copenhagen S (DK) Designated Extension States: • KALDOR, Ditte Kievsmose AL BA HR MK RS DK-2880 Bagsvaerd (DK) • SLØK, Frank Abilgaard (30) Priority: 01.12.2005 DK 200501704 DK-3450 Allerød (DK) 02.12.2005 US 741490 P • HUSEMOEN, Birgitte Nystrup DK-2500 Valby (DK) (43) Date of publication of application: • DOLBERG, Johannes 20.08.2008 Bulletin 2008/34 DK-1674 Copenhagen V (DK) • JENSEN, Kim Birkebæk (73) Proprietor: Nuevolution A/S DK-2610 Rødovre (DK) 2100 Copenhagen 0 (DK) • PETERSEN, Lene DK-2100 Copenhagen Ø (DK) (72) Inventors: • NØRREGAARD-MADSEN, Mads • FRANCH, Thomas DK-3460 Birkerød (DK) DK-3070 Snekkersten (DK) • GODSKESEN, -

PROTEOMIC ANALYSIS of HUMAN URINARY EXOSOMES. Patricia
ABSTRACT Title of Document: PROTEOMIC ANALYSIS OF HUMAN URINARY EXOSOMES. Patricia Amalia Gonzales Mancilla, Ph.D., 2009 Directed By: Associate Professor Nam Sun Wang, Department of Chemical and Biomolecular Engineering Exosomes originate as the internal vesicles of multivesicular bodies (MVBs) in cells. These small vesicles (40-100 nm) have been shown to be secreted by most cell types throughout the body. In the kidney, urinary exosomes are released to the urine by fusion of the outer membrane of the MVBs with the apical plasma membrane of renal tubular epithelia. Exosomes contain apical membrane and cytosolic proteins and can be isolated using differential centrifugation. The analysis of urinary exosomes provides a non- invasive means of acquiring information about the physiological or pathophysiological state of renal cells. The overall objective of this research was to develop methods and knowledge infrastructure for urinary proteomics. We proposed to conduct a proteomic analysis of human urinary exosomes. The first objective was to profile the proteome of human urinary exosomes using liquid chromatography-tandem spectrometry (LC- MS/MS) and specialized software for identification of peptide sequences from fragmentation spectra. We unambiguously identified 1132 proteins. In addition, the phosphoproteome of human urinary exosomes was profiled using the neutral loss scanning acquisition mode of LC-MS/MS. The phosphoproteomic profiling identified 19 phosphorylation sites corresponding to 14 phosphoproteins. The second objective was to analyze urinary exosomes samples isolated from patients with genetic mutations. Polyclonal antibodies were generated to recognize epitopes on the gene products of these genetic mutations, NKCC2 and MRP4. The potential usefulness of urinary exosome analysis was demonstrated using the well-defined renal tubulopathy, Bartter syndrome type I and using the single nucleotide polymorphism in the ABCC4 gene. -

Cloud-Clone 16-17
Cloud-Clone - 2016-17 Catalog Description Pack Size Supplier Rupee(RS) ACB028Hu CLIA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 74750 AEA044Hu ELISA Kit for Anti-Growth Hormone Antibody (Anti-GHAb) 96T Cloud-Clone 74750 AEA255Hu ELISA Kit for Anti-Apolipoprotein Antibodies (AAHA) 96T Cloud-Clone 74750 AEA417Hu ELISA Kit for Anti-Proteolipid Protein 1, Myelin Antibody (Anti-PLP1) 96T Cloud-Clone 74750 AEA421Hu ELISA Kit for Anti-Myelin Oligodendrocyte Glycoprotein Antibody (Anti- 96T Cloud-Clone 74750 MOG) AEA465Hu ELISA Kit for Anti-Sperm Antibody (AsAb) 96T Cloud-Clone 74750 AEA539Hu ELISA Kit for Anti-Myelin Basic Protein Antibody (Anti-MBP) 96T Cloud-Clone 71250 AEA546Hu ELISA Kit for Anti-IgA Antibody 96T Cloud-Clone 71250 AEA601Hu ELISA Kit for Anti-Myeloperoxidase Antibody (Anti-MPO) 96T Cloud-Clone 71250 AEA747Hu ELISA Kit for Anti-Complement 1q Antibody (Anti-C1q) 96T Cloud-Clone 74750 AEA821Hu ELISA Kit for Anti-C Reactive Protein Antibody (Anti-CRP) 96T Cloud-Clone 74750 AEA895Hu ELISA Kit for Anti-Insulin Receptor Antibody (AIRA) 96T Cloud-Clone 74750 AEB028Hu ELISA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 71250 AEB264Hu ELISA Kit for Insulin Autoantibody (IAA) 96T Cloud-Clone 74750 AEB480Hu ELISA Kit for Anti-Mannose Binding Lectin Antibody (Anti-MBL) 96T Cloud-Clone 88575 AED245Hu ELISA Kit for Anti-Glutamic Acid Decarboxylase Antibodies (Anti-GAD) 96T Cloud-Clone 71250 AEK505Hu ELISA Kit for Anti-Heparin/Platelet Factor 4 Antibodies (Anti-HPF4) 96T Cloud-Clone 71250 CCA005Hu CLIA Kit for Angiotensin II -

The Role of Amino Acids in Liver Protein Metabolism Under a High Protein Diet
The role of amino acids in liver protein metabolism under a high protein diet : identification of amino acids signal and associated transduction pathways Nattida Chotechuang To cite this version: Nattida Chotechuang. The role of amino acids in liver protein metabolism under a high protein diet : identification of amino acids signal and associated transduction pathways. Food and Nutrition. AgroParisTech, 2010. English. NNT : 2010AGPT0026. pastel-00610998 HAL Id: pastel-00610998 https://pastel.archives-ouvertes.fr/pastel-00610998 Submitted on 25 Jul 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° /__/__/__/__/__/__/__/__/__/__/ T H E S I S submitted to obtain the degree of Doctor of Philosophy at L’Institut des Sciences et Industries du Vivant et de l’Environnement (AgroParisTech) Speciality: Nutrition Science Presented and defended in public by Nattida CHOTECHUANG on 22nd March 2010 THE ROLE OF AMINO ACIDS IN LIVER PROTEIN METABOLISM UNDER A HIGH PROTEIN DIET: IDENTIFICATION OF AMINO ACIDS SIGNAL AND ASSOCIATED TRANSDUCTION PATHWAYS Thesis director: Daniel TOMÉ Thesis co-director: Dalila AZZOUT-MARNICHE AgroParisTech, UMR914 Nutrition Physiology and Ingestive Behaviour, F-75005 Paris to the jury: Mr. -

Characterization of Aminopeptidase in the Free-Living Nematode Panagrellus Redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E
Journal of Nematology 39(2):153–160. 2007. © The Society of Nematologists 2007. Characterization of Aminopeptidase in the Free-living Nematode Panagrellus redivivus: Subcellular Distribution and Possible Role in Neuropeptide Metabolism E. P. Masler Abstract: Aminopeptidase was detected in homogenates of the free-living nematode Panagrellus redivivus with the aminoacyl substrate L-alanine-4-nitroanilide. Subcellular distribution of activity was 80% soluble and 20% membrane-associated. Aminopep- tidases in the two fractions differed in affinity for Ala-4-NA, with Km’s of 0.65 mM (soluble) and 2.90 mM (membrane). Specific activities (units/mg) at pH 7.8, 27°C were 9.10 (soluble) and 14.30 (membrane). Each enzyme was competitively inhibited by amastatin (90% at 100 µM inhibitor, IC50 = 3.7 µM) and inhibited by puromycin (30% at 500 µM) and 1,10-phenanthroline (IC50’s:; 148 µM, soluble; 89 µM, membrane). Activity was restored by Zn++, with maximum recoveries of 50% (soluble) and 90% (mem- ∼ brane), each at 23 µM ZnCl2. Estimated molecular masses for each were 150 kDa. FMRFamide-like neuropeptides behaved as competitive inhibitors. Modification of the N-terminal F of FMRFamide weakened inhibition by 95%, suggesting that the N-terminus is essential for binding to the enzyme. Two nematode FMRFamides, APKPFIRFa and RNKFEFIRFa, were the most potent tested. This is the first biochemical characterization of aminopeptidase in a free-living nematode other than Caenorhabditis elegans and demon- strates the high selectivity of the P. redivivus enzymes for neuropeptide substrates. Key words: FMRFamide-like peptide, inhibitor; membrane, metallopeptidase, neuropeptide, protease Nematodes, like other eukaryotic organisms, depend reproduction (Day and Maule, 1999; Maule et al., 2002; upon proteolytic enzymes for the regulation of essential Rogers et al., 2003). -

Code Lists Page 1
Code lists Page 1 Code lists AESEV Page 2 AESEV Codelist Name: Severity/Intensity Scale for Adverse Events Description: A scale that defines the degree or state of disease existing in a patient as a result of the occurrence of an adverse event. (NCI) C41338,1; Grade 1 C41339,2; Grade 2 C41340,3; Grade 3 AGEU Page 3 AGEU Codelist Name: Age Unit Description: Those units of time that are routinely used to express the age of a subject. C25301,Days C25529,Hours; h; hr C29846,Month C29844,Week C29848,Year CMDOSFRM Page 4 CMDOSFRM Codelist Name: Concomitant Medication Dose Form Description: A terminology subset of the CDISC SDTM Pharmaceutical Dosage Form codelist created for CDASH Concomitant Medication Dose Form codelist. (NCI) C42887,AEROSOL; aer C25158,CAPSULE; cap C28944,CREAM; C42933,GAS; C42934,GEL; C42966,OINTMENT; oint C42968,PATCH; C42972,POWDER; C42989,SPRAY; C42993,SUPPOSITORY; supp C42994,SUSPENSION; susp C42998,TABLET; tab CMDOSFRQ Page 5 CMDOSFRQ Codelist Name: Concomitant Medication Dosing Frequency per Interval Description: A terminology subset of the CDISC SDTM Frequency codelist created for CDASH Concomitant Medication Dosing Frequency per Interval codelist. (NCI) C64496,BID; BD; Twice per day C64499,PRN; As needed C25473,QD; Daily C64530,QID; 4 times per day C64498,QM; Every Month; Per Month C64525,QOD; Every other day C64527,TID; 3 times per day C17998,UNKNOWN; U; UNK; Unknown CMDOSU Page 6 CMDOSU Codelist Name: Concomitant Medication Dose Units Description: A terminology subset of the CDISC SDTM Unit codelist created for CDASH Concomitant Medication Dose Units codelist. (NCI) C48480,CAPSULE; Capsule Dosing Unit; cap C48155,g; Gram C48579,IU; IE; International Unit C28253,mg; Milligram C28254,mL; Milliliter; cm3 C65060,PUFF; Puff Dosing Unit C48542,TABLET; Tablet Dosing Unit; tab C48152,ug; Microgram; mcg CMROUTE Page 7 CMROUTE Codelist Name: Concomitant Medication Route of Administration Description: A terminology subset of the CDISC SDTM Route codelist created for CDASH Concomitant Medication Route of Administration codelist. -

REVIEW ARTICLE High Molecular Mass Intracellular Proteases
Biochem J. (1989) 263, 625-633 (Printed in Great Britain) 625 REVIEW ARTICLE High molecular mass intracellular proteases A. Jennifer RIVETT Department of Biochemistry, University of Leicester, Leicester LE'l 7RH, U.K. INTRODUCTION demonstrated that intracellular proteolysis is not re- Many of the well-characterized proteolytic enzymes, stricted to the lysosomes. Since a large proportion of and particularly those for which X-ray structures are intracellular protein breakdown, especially the degra- now available, are small monomeric enzymes often dation of proteins with short half-lives, is now known to having molecular masses in the range of 20-30 kDa. occur by nonlysosomal mechanisms (Mayer & Doherty, Many of them are extracellular enzymes which are easy 1986; Bond & Beynon, 1987; Rechsteiner, 1987; Bohley, to assay and to purify. With a growing awareness of the 1987; Rivett, 1989b; Katunuma & Kominami, 1989; importance of intracellular protein turnover and Knecht & Grisolia, 1989), there is now a greater interest mechanisms of intracellular protein breakdown, interest in nonlysosomal degradation systems and in nonlyso- in the proteases responsible has also increased. Although somal proteinases, many of which have large complex some intracellular proteases, especially those found structures. within the lysosomes in animal cells, are, like extracellular In contrast to the well-known lysosomal proteases, proteases, small and highly active monomeric enzymes, soluble extralysosomal proteases often have multimeric a number of cellular proteases -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -
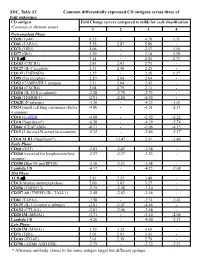
SDC, Table S1. Common Differentially Expressed CD Antigens
SDC, Table S1. Common differentially expressed CD antigens across three of four outcomes CD antigen Fold Change (severe compared to mild) for each classification (Common or alternate name) 1 2 3 4 Pretransplant Phase CD28 (Tp44) 6.32 - 4.79 3.71 CD81 (TAPA1) 5.58 2.87 5.86 - CD71 (TfR1) 4.06 - 2.57 3.16 CD77 (Gb3) 3.29 - 3.18 3.29 TCR ab 3.14 - 2.01 2.73 CD183 (CXCR3) 2.43 2.93 2.71 - CD127 (IL-7 receptor) 2.31 2.48 2.25 - CD137 (TNFRSF9) 2.27 - 2.35 2.27 CD95 (Fas receptor) 2.20 2.04 2.64 - CD52 (CAMPATH-1 antigen) 2.11 2.04 2.43 - CD184 (CXCR4) 2.04 2.79 2.11 - CD210 (IL-10 R a subunit) -2.28 -2.79 -2.73 - CD40 (TNFRSF5) -2.91 -2.20 -4.92 - CD62E (E-selectin) -3.36 - -4.23 -3.01 CD24 (small cell lung carcinoma cluster -4.00 - -3.51 -2.33 4 antigen) CD16 (FcγRIII) -4.08 - -2.92 -2.22 CD10 (Neprilysin*) -6.28 - -4.29 -5.74 CD66c (CEACAM6) -8.51 - -5.06 -6.15 CD15 (3-fucosyl-N-acetyl-lactosamine) -9.32 - -5.86 -5.17 CD10 ALB1 (Neprilysin*) - 12.47 2.51 -3.46 Early Phase CD60 (GD3) -2.03 -2.43 -2.58 - CD260 (reserved for lymphotoxin beta -2.57 -2.27 -3.23 - receptor) CD180 (Bgp-95 and RP105) -3.10 -2.33 -3.48 - Lambda 1/8 -4.17 - -4.23 -2.68 Mid Phase TCR ab 2.81 2.22 3.48 - CD13(Alanine aminopeptidase) 2.00 3.03 3.27 - CD256 (TNFSF13) -2.19 -2.48 -3.10 - CD257 AS (TNFSF13B / TALL1) -2.48 -2.62 -3.46 - CD81 (TAPA1) -2.58 - -2.31 -2.43 CD125 (IL-5 receptor a subunit) -2.81 -3.07 -4.66 - CD152 (CTLA-4) -2.81 -2.06 -3.48 - CD20 IM (MS4A1) -3.71 - -3.10 -2.04 Lambda 1/8 -4.26 - -4.00 -2.13 Late Phase CD20 IM (MS4A1) 3.39 3.32 4.69 - CD71 (TfR1) 2.03 2.93 3.01 - CD103 (ITGAE) 2.03 2.39 2.79 - CD79b (AGM6 AND IGB) -2.79 - -2.32 -2.23 * Alternate antibody clones to the same antigen target but different epitope. -

©Ferrata Storti Foundation
Original Articles T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolerogenic host immune response Peter Van Loo,1,2,3 Thomas Tousseyn,4 Vera Vanhentenrijk,4 Daan Dierickx,5 Agnieszka Malecka,6 Isabelle Vanden Bempt,4 Gregor Verhoef,5 Jan Delabie,6 Peter Marynen,1,2 Patrick Matthys,7 and Chris De Wolf-Peeters4 1Department of Molecular and Developmental Genetics, VIB, Leuven, Belgium; 2Department of Human Genetics, K.U.Leuven, Leuven, Belgium; 3Bioinformatics Group, Department of Electrical Engineering, K.U.Leuven, Leuven, Belgium; 4Department of Pathology, University Hospitals K.U.Leuven, Leuven, Belgium; 5Department of Hematology, University Hospitals K.U.Leuven, Leuven, Belgium; 6Department of Pathology, The Norwegian Radium Hospital, University of Oslo, Oslo, Norway, and 7Department of Microbiology and Immunology, Rega Institute for Medical Research, K.U.Leuven, Leuven, Belgium Citation: Van Loo P, Tousseyn T, Vanhentenrijk V, Dierickx D, Malecka A, Vanden Bempt I, Verhoef G, Delabie J, Marynen P, Matthys P, and De Wolf-Peeters C. T-cell/histiocyte-rich large B-cell lymphoma shows transcriptional features suggestive of a tolero- genic host immune response. Haematologica. 2010;95:440-448. doi:10.3324/haematol.2009.009647 The Online Supplementary Tables S1-5 are in separate PDF files Supplementary Design and Methods One microgram of total RNA was reverse transcribed using random primers and SuperScript II (Invitrogen, Merelbeke, Validation of microarray results by real-time quantitative Belgium), as recommended by the manufacturer. Relative reverse transcriptase polymerase chain reaction quantification was subsequently performed using the compar- Ten genes measured by microarray gene expression profil- ative CT method (see User Bulletin #2: Relative Quantitation ing were validated by real-time quantitative reverse transcrip- of Gene Expression, Applied Biosystems). -

Companion Animals: Nutrition & Health
T28 Relationship between calpastatin gene polymorphism and T29 Corn oil or Corn grain supplementation to forage-nished beef cattle growth, carcass and meat quality traits. L. Suguisawa, steers. IV. Effects on gene expression of lipogenic enzymes in the A. A. Souza*, H. N. Oliveira, A. C. Silveira, and R. A. Cury, São s.c. adipose tissue. E. Pavan*1,2, S. Joseph1, K. Robbins1, S. Duckett3, Paulo State University, Brazil. and R. Rekaya1, 1University of Georgia, Athens, 2INTA, Balcarce, Bs. As., Arg., 3Clemson University, Clemson, SC. The Calpastatin polymorphism was associated with performance and meat quality traits, by Chung et al. (2001) methodology, in 300 Samples of s.c. fat were obtained from 28 Angus steers after slaughter bullocks. To validate this nding, we looked at a group of animals to determine the effect of energy supplementation of steers grazing (126 Angus Nellore, 10 Angus, 18 Brangus, 24 Simmental Nellore, tall fescue pastures. Steers (n = 8/ treatment; 289 ± 3.8 kg) were 12 Simental, 11 Simbrasil, 17 Santa Gertrudes Nellore, 18 Brown supplemented with either corn grain (0.52% BW; PC) or soybean hulls Swiss x Nelore, 12 Canchim, 16 Brahman Nellore and 36 Nellore). plus corn oil (0.45% BW + 0.10% corn oil; PO). Negative (pasture The animals were weaned at 7 months old at the creep-feeding and only; P) and positive (85% concentrate/15% roughage; C) controls raised at the feedlot system for 120 days. The animals were harvested were also included in the study. RNA was extracted from the s.c. with 450 kg live weight, 3 millimeters of ultrasound fat thickness adipose tissue using TRIzol reagent (Gibco Invitrogen Corp.).