Report of the International Narcotics Control Board for 2012 (E/INCB/2012/1) Is Supplemented by the Following Reports
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Report of the International Narcotics Control Board for 2012
CHAPTER III. ANALYSIS OF THE WORLD SITUATION reported slight increases, however, while other acceding to the other two international drug control countries reported increases in injecting risk behaviour treaties. or low coverage of prevention services among injecting 803. However, the fact remains that nine States in drug users. Oceania have yet to become parties to all three of the international drug control treaties, and this continues to E. Oceania be a matter of grave concern for the Board, particularly in the light of increased reports of trafficking in and illicit 1. Major developments manufacture of drugs in the region. High prevalence rates for the abuse of cannabis and knowledge of illicit 800. The rates of abuse and illicit manufacture of methamphetamine manufacture in Oceania make it an amphetamine-type stimulants in Oceania are still among area particularly susceptible to organized crime. The the highest in the world. This trend is particularly well Board continues to urge all States concerned, namely the documented in Australia and New Zealand, although methamphetamine abuse is reported to be stable or Cook Islands, Kiribati, Nauru, Palau, Papua New Guinea, declining in those countries. While domestic illicit Samoa, Solomon Islands, Tuvalu and Vanuatu, to accede manufacture in Australia and New Zealand is widespread, without further delay to any of the three international the recent crackdown on precursor chemicals used in drug control treaties to which they are not yet parties. domestic manufacture has caused the price of Those States may easily become used by traffickers who amphetamine-type stimulants to rise, which has in turn want to supply the Australian and New Zealand markets. -

DEMAND REDUCTION a Glossary of Terms
UNITED NATIONS PUBLICATION Sales No. E.00.XI.9 ISBN: 92-1-148129-5 ACKNOWLEDGEMENTS This document was prepared by the: United Nations International Drug Control Programme (UNDCP), Vienna, Austria, in consultation with the Commonwealth of Health and Aged Care, Australia, and the informal international reference group. ii Contents Page Foreword . xi Demand reduction: A glossary of terms . 1 Abstinence . 1 Abuse . 1 Abuse liability . 2 Action research . 2 Addiction, addict . 2 Administration (method of) . 3 Adverse drug reaction . 4 Advice services . 4 Advocacy . 4 Agonist . 4 AIDS . 5 Al-Anon . 5 Alcohol . 5 Alcoholics Anonymous (AA) . 6 Alternatives to drug use . 6 Amfetamine . 6 Amotivational syndrome . 6 Amphetamine . 6 Amyl nitrate . 8 Analgesic . 8 iii Page Antagonist . 8 Anti-anxiety drug . 8 Antidepressant . 8 Backloading . 9 Bad trip . 9 Barbiturate . 9 Benzodiazepine . 10 Blood-borne virus . 10 Brief intervention . 11 Buprenorphine . 11 Caffeine . 12 Cannabis . 12 Chasing . 13 Cocaine . 13 Coca leaves . 14 Coca paste . 14 Cold turkey . 14 Community empowerment . 15 Co-morbidity . 15 Comprehensive Multidisciplinary Outline of Future Activities in Drug Abuse Control (CMO) . 15 Controlled substance . 15 Counselling and psychotherapy . 16 Court diversion . 16 Crash . 16 Cross-dependence . 17 Cross-tolerance . 17 Custody diversion . 17 Dance drug . 18 Decriminalization or depenalization . 18 Demand . 18 iv Page Demand reduction . 19 Dependence, dependence syndrome . 19 Dependence liability . 20 Depressant . 20 Designer drug . 20 Detoxification . 20 Diacetylmorphine/Diamorphine . 21 Diuretic . 21 Drug . 21 Drug abuse . 22 Drug abuse-related harm . 22 Drug abuse-related problem . 22 Drug policy . 23 Drug seeking . 23 Drug substitution . 23 Drug testing . 24 Drug use . -

BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18
Oregon Board of Pharmacy BOARD MEETING AGENDA Meeting Location: Portland State Office Building 800 NE Oregon Street, Portland, OR 97232 June 6-7, 2018 Updated 6.4.18 The mission of the Oregon State Board of Pharmacy is to promote, preserve and protect the public health, safety and welfare by ensuring high standards in the practice of pharmacy and by regulating the quality, manufacture, sale and distribution of drugs. Wednesday, June 6, 2018 @ 8:30AM – Conference Rm A Thursday, June 7, 2018 @ 8:30AM – Conference Room A ≈ If special accommodations are needed for you to attend or participate in this Board Meeting, please contact Loretta Glenn at: (971) 673-0001. ≈ WEDNESDAY, JUNE 6, 2018 I. 8:30AM OPEN SESSION, Penny Reher, R.Ph, Presiding A. Roll Call B. Agenda Review and Approval Action Necessary II. Contested Case Deliberation pursuant to ORS 192.690(1) - Not Open to the Public III. EXECUTIVE SESSION – NOT OPEN TO THE PUBLIC, pursuant to ORS 676.175, ORS 192.660 (1) (2) (f) (k). A. Items for Consideration and Discussion: 1. Deliberation on Disciplinary Cases and Investigations 2. Personal Appearances 3. Deficiency Notifications 4. Case Review B. Employee Performance Review pursuant to ORS 192.660(2)(i). IV. OPEN SESSION - PUBLIC MAY ATTEND - At the conclusion of Executive Session, the Board may convene Open Session to begin some of the following scheduled agenda items - time permitting at approximately 3:30PM. V. Approve Consent Agenda* Action Necessary *Items listed under the consent agenda are considered to be routine agency matters and will be approved by a single motion of the Board without separate discussion. -
Prezentace Aplikace Powerpoint
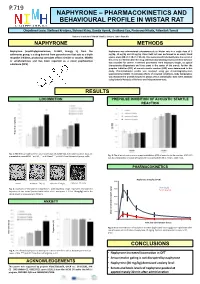
P.719 NAPHYRONE – PHARMACOKINETICS AND BEHAVIOURAL PROFILE IN WISTAR RAT Olejíková Lucie, Štefková Kristýa, Šíchová Klára, Dada Hyek, Lhotková Eva, Piterová Nikola, Páleíček Toáš National Institute of Mental Health, Klecany, Czech Republic NAPHYRONE METHODS Naphyrone (naphthylpyrovalerone, O-2482, Energy 1), from the Naphyrone was administered subcutaneously to Wistar rats in a single dose of 5 cathinones group, is a drug derived from pyrovalerone that acts as a triple mg/kg, 10 mg/kg and 20 mg/kg. Open field test was performed in an empty black reuptake inhibitor, producing stimulant effects similar to cocaine, MDMA square arena (68 c × 68 c × 30 c). Rats were placed individually into the center of or amphetamines and has been reported as a novel psychoactive the arena 5 or 40 i after the drug administration (testing-onset) and their behavior was recorded for 30 i. Examined parameters were trajectory length, its spatial substance (NPS). characteristic (thigmotaxis and time spent in the center of the arena). Further the prepulse inhibition (PPI) of acoustic startle reaction (ASR) were determined in this study. Pharmacokinetic profile was analyzed using gas chromatography-mass spectrometry (GCMS). To simulate effects of crowded conditions, body temperature was monitored in animals housed in groups versus individually. Data were analyzed using factorial Analysis of Variance and independent t-tests. RESULTS LOCOMOTION PREPULSE INHIBITION OF ACOUSTIC STARTLE REACTION lenght (m) Trajectory Fig. 1: The effect of naphyrone on total locomotion 15 and 60 min after administration. Data are Fig. 3: The effect of naphyrone on prepulse inhibition (PPI) of acoustic startle reaction (ASR). PPI presented as mean±SEM. -

New Zealand's Emergent Opioid Trends
New Zealand’s emergent opioid trends - the influences and impacts: Consumer and clinician understanding of New Zealand’s changing patterns of opioid use, availability and impacts Klare Braye A thesis submitted in partial fulfilment of the requirements for the degree of Master of Health Sciences Department of Psychological Medicine University of Otago, Christchurch New Zealand 2014 “Among the remedies which it has pleased Almighty God to give to man to relieve his sufferings, none is so universal and so efficacious as opium”. (Sir Thomas Sydenham, 1680) ~ iii ~ Abstract This thesis explores opioid users and addiction service providers’ understandings of the changing patterns, availability and impacts of opioids and opioid use in New Zealand with a particular focus on the last two decades. Throughout history, the availability of substances of abuse have waxed and waned. Their emergence and availability is influenced by social, legislative and political circumstances. Extensive literature exists related to the use of opioids such as morphine and methadone in New Zealand, and heroin overseas. However, few studies have examined the emerging trends of opioids that are often used as adjunctively to these substances; examples of which in recent years include poppy seed tea (PST), over-the- counter codeine-containing analgesics and most recently some prescribed pain medications. Information regarding the use, availability and impacts of these adjunct opioids from the consumers who use them, and the clinicians who work with these consumers could usefully inform service provision and public policy. This qualitative study, adopting a Husserlian phenomenological philosophy was used, to gain an understanding of the use, access to and implications of these emergent opioid trends. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Author(s): Peter Stout, Katherine Moore, Megan Grabenauer, Jeri Ropero-Miller Document No.: 241444 Date Received: March 2013 Award Number: 2010-DN-BX-K177 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Award Number: 2010-DN-BX-K177 July 16, 2012 Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Final Report Authors: Peter Stout Katherine Moore Megan Grabenauer Jeri Ropero-Miller This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Expansion of a Cheminformatic Database of Spectral Data for Forensic Chemists and Toxicologists Table of Contents Abstract ........................................................................................................................................... 1 Executive Summary ....................................................................................................................... -

Recent Trends in Illegal Drug Use in New Zealand, 2005-2007
RECENT TRENDS IN ILLEGAL DRUG USE IN NEW ZEALAND, 2005-2007 Findings from the 2005, 2006 and 2007 Illicit Drug Monitoring System (IDMS) C.Wilkins M. Girling P. Sweetsur Centre for Social and Health Outcomes Research and Evaluation Massey University, P O Box 6137, Wellesley St February 2008 © Centre for Social and Health Outcomes Research and Evaluation ISBN 1 877428 07 8 Table of Contents Acknowledgements............................................................................................................................. 8 Executive Summary ............................................................................................................................ 9 Summary ........................................................................................................................................... 11 Introduction....................................................................................................................................11 Method ...........................................................................................................................................11 Demographic characteristics of the frequent drug users................................................................12 Drug use patterns of the frequent methamphetamine users ...........................................................13 Drug use patterns of the frequent ecstasy users .............................................................................14 Drug use patterns of the frequent injecting drug users ..................................................................14 -

Dextropropoxyphene/Diacerein 41 Precautions the Elderly
Dextropropoxyphene/Diacerein 41 Precautions The elderly. The elimination half-lives of dextropropoxyphene 3. Haigh S. 12 Years on: co-proxamol revisited. Lancet 1996; 347: As for Opioid Analgesics in general, p.103. and its metabolite nordextropropoxyphene were prolonged in 1840–1. Correction. ibid.; 348: 346. healthy elderly subjects when compared with young controls.1 4. Sykes JV, et al. Coproxamol revisited. Lancet 1996; 348: 408. Abuse. There have been reports1 of the abuse of dextropropox- After multiple dosing median half-lives of dextropropoxyphene 5. Li Wan Po A, Zhang WY. Systematic overview of co-proxamol 2 to assess analgesic effects of addition of dextropropoxyphene to yphene, and some considered that the ready availability of dex- and nordextropropoxyphene were 36.8 and 41.8 hours, respec- paracetamol. BMJ 1997; 315: 1565–71. Correction ibid. 1998; tropropoxyphene made it liable to abuse although it was a rela- tively in the elderly compared with 22.0 and 22.1 hours in the 316: 116 and 656. tively weak opioid analgesic. However, others3 thought there young subjects. In this study1 there was a strong correlation be- 6. Anonymous. Co-proxamol or paracetamol for acute pain? Drug was no evidence that dextropropoxyphene was frequently asso- tween half-life of nordextropropoxyphene and estimated creati- Ther Bull 1998; 36: 80. ciated with abuse, or concluded that, although there was abuse nine clearance. 7. MHRA. Withdrawal of co-proxamol products and interim updat- ed prescribing information. Message from Professor G Duff, potential, it was of relatively low importance in terms of the com- 1. Flanagan RJ, et al. -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens. -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: