Geographic Variation, Genetic Structure, and Conservation Unit Designation in the Larch Mountain Salamander (Plethodon Larselli)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guide to Priority Plant and Animal Species in Oregon Forests
A GUIDE TO Priority Plant and Animal Species IN OREGON FORESTS A publication of the Oregon Forest Resources Institute Sponsors of the first animal and plant guidebooks included the Oregon Department of Forestry, the Oregon Department of Fish and Wildlife, the Oregon Biodiversity Information Center, Oregon State University and the Oregon State Implementation Committee, Sustainable Forestry Initiative. This update was made possible with help from the Northwest Habitat Institute, the Oregon Biodiversity Information Center, Institute for Natural Resources, Portland State University and Oregon State University. Acknowledgments: The Oregon Forest Resources Institute is grateful to the following contributors: Thomas O’Neil, Kathleen O’Neil, Malcolm Anderson and Jamie McFadden, Northwest Habitat Institute; the Integrated Habitat and Biodiversity Information System (IBIS), supported in part by the Northwest Power and Conservation Council and the Bonneville Power Administration under project #2003-072-00 and ESRI Conservation Program grants; Sue Vrilakas, Oregon Biodiversity Information Center, Institute for Natural Resources; and Dana Sanchez, Oregon State University, Mark Gourley, Starker Forests and Mike Rochelle, Weyerhaeuser Company. Edited by: Fran Cafferata Coe, Cafferata Consulting, LLC. Designed by: Sarah Craig, Word Jones © Copyright 2012 A Guide to Priority Plant and Animal Species in Oregon Forests Oregonians care about forest-dwelling wildlife and plants. This revised and updated publication is designed to assist forest landowners, land managers, students and educators in understanding how forests provide habitat for different wildlife and plant species. Keeping forestland in forestry is a great way to mitigate habitat loss resulting from development, mining and other non-forest uses. Through the use of specific forestry techniques, landowners can maintain, enhance and even create habitat for birds, mammals and amphibians while still managing lands for timber production. -

Aneides Vagrans Residing in the Canopy of Old-Growth Redwood Forest 1 1 1 James C
Herpetological Conservation and Biology 1(1):16-26 Submitted: June 15, 2006; Accepted: July 19, 2006 EVIDENCE OF A NEW NICHE FOR A NORTH AMERICAN SALAMANDER: ANEIDES VAGRANS RESIDING IN THE CANOPY OF OLD-GROWTH REDWOOD FOREST 1 1 1 JAMES C. SPICKLER , STEPHEN C. SILLETT , SHARYN B. MARKS , 2,3 AND HARTWELL H. WELSH, JR. 1Department of Biological Sciences, Humboldt State University, Arcata, CA 95521, USA 2Redwood Sciences Laboratory, USDA Forest Service, 1700 Bayview Drive, Arcata, CA 95521, USA 3Corresponding Author, email: [email protected] Abstract.—We investigated habitat use and movements of the wandering salamander, Aneides vagrans, in an old-growth forest canopy. We conducted a mark-recapture study of salamanders in the crowns of five large redwoods (Sequoia sempervirens) in Prairie Creek Redwoods State Park, California. This represented a first attempt to document the residency and behavior of A. vagrans in a canopy environment. We placed litter bags on 65 fern (Polypodium scouleri) mats, covering 10% of their total surface area in each tree. Also, we set cover boards on one fern mat in each of two trees. We checked cover objects 2–4 times per month during fall and winter seasons. We marked 40 individuals with elastomer tags and recaptured 13. Only one recaptured salamander moved (vertically 7 m) from its original point of capture. We compared habitats associated with salamander captures using correlation analysis and stepwise regression. At the tree-level, the best predictor of salamander abundance was water storage by fern mats. At the fern mat-level, the presence of cover boards accounted for 85% of the variability observed in captures. -

Management Indicator Species Review
Management Indicator Species Review Public Wheeled Motorized Travel Management Six Rivers National Forest Mad River and Lower Trinity Ranger District March 2009 July 20, 2009 edits Jan. 15, 2010 edits Prepared by: ____Kary Schick____________ Date: ___July 7, 2009____________ Kary E. Schlick Wildlife Biologist Prepared by: ____Karen Kenfield__________ Date: ___July 7, 2009____________ Karen Kenfield Fisheries Biologist Introduction Under the National Forest Management Act (NFMA), the Forest Service is directed to “provide for diversity of plant and animal communities based on the suitability and capability of the specific land area in order to meet overall multiple-use objectives.” (P.L. 94-588, Sec 6 (g) (3) (B)). The 1982 regulations implementing NFMA require that “Fish and wildlife habitat shall be managed to maintain viable populations of existing native and desired non-native vertebrate species in the planning area.” (36 CFR 219.19) Management Indicator Species (MIS) is a concept used by the agency to serve as a barometer for species viability at the Forest level. The Six Rivers National Forest Land and Resource Management Plan (LRMP) uses Management Indicator Species (MIS) to assess potential effects of management activities on the various habitats and habitat assemblages with which these species are associated. There are seven habitat assemblages containing 41 fish and wildlife species on the Forest (LRMP IV-97). Detailed descriptions of the Travel Management alternatives are found in the Travel Management Environmental Impact Statement. For the analysis associated with this project, specific MIS were addressed based on their potential to occur within the Travel Management planning. Table 1 lists the MIS and assemblages occurring on the Six Rivers National Forest, and those known or thought to occur within the planning area based on habitat suitability, survey results, or incidental sighting records. -

Diet of the Del Norte Salamander (Plethodon Elongatus): Differences by Age, Gender, and Season
NORTHWESTERN NATURALIST 88:85–94 AUTUMN 2007 DIET OF THE DEL NORTE SALAMANDER (PLETHODON ELONGATUS): DIFFERENCES BY AGE, GENDER, AND SEASON CLARA AWHEELER,NANCY EKARRAKER1,HARTWELL HWELSH,JR, AND LISA MOLLIVIER Redwood Sciences Laboratory, Pacific Southwest Research Station, USDA Forest Service, 1700 Bayview Drive, Arcata, California 95521 ABSTRACT—Terrestrial salamanders are integral components of forest ecosystems and the ex- amination of their feeding habits may provide useful information regarding various ecosystem processes. We studied the diet of the Del Norte Salamander (Plethodon elongatus) and assessed diet differences between age classes, genders, and seasons. The stomachs of 309 subadult and adult salamanders, captured in spring and fall, contained 20 prey types. Nineteen were inver- tebrates, and one was a juvenile Del Norte Salamander, representing the first reported evidence of cannibalism in this species. Mites and ants represented a significant component of the diet across all age classes and genders, and diets of subadult and adult salamanders were fairly similar overall. We detected, however, an ontogenetic shift with termites and ants becoming less important and spiders and mites becoming more important with age. These differences between subadults and adults can likely be attributed to the inability of subadults to consume larger prey items due in part to gape limitation. The diet of the Del Norte Salamander, like other plethodontids, consists of a high diversity of prey items making it an opportunistic, sit-and- wait predator. Key words: Del Norte Salamander, Plethodon elongatus, food habits, diet, northern California, southern Oregon Terrestrial salamanders represent a signifi- 2005), and may require ecological conditions cant component of vertebrate biomass in forest found primarily in late seral stage forests ecosystems (Burton and Likens 1975a) and (Welsh 1990; Welsh and Lind 1995; Jones and strongly influence nutrient dynamics and en- others 2005). -

Amphibian Taxon Advisory Group Regional Collection Plan
1 Table of Contents ATAG Definition and Scope ......................................................................................................... 4 Mission Statement ........................................................................................................................... 4 Addressing the Amphibian Crisis at a Global Level ....................................................................... 5 Metamorphosis of the ATAG Regional Collection Plan ................................................................. 6 Taxa Within ATAG Purview ........................................................................................................ 6 Priority Species and Regions ........................................................................................................... 7 Priority Conservations Activities..................................................................................................... 8 Institutional Capacity of AZA Communities .............................................................................. 8 Space Needed for Amphibians ........................................................................................................ 9 Species Selection Criteria ............................................................................................................ 13 The Global Prioritization Process .................................................................................................. 13 Selection Tool: Amphibian Ark’s Prioritization Tool for Ex situ Conservation .......................... -

Rare, Threatened and Endangered Species of Oregon
Portland State University PDXScholar Institute for Natural Resources Publications Institute for Natural Resources - Portland 8-2016 Rare, Threatened and Endangered Species of Oregon James S. Kagan Portland State University Sue Vrilakas Portland State University, [email protected] John A. Christy Portland State University Eleanor P. Gaines Portland State University Lindsey Wise Portland State University See next page for additional authors Follow this and additional works at: https://pdxscholar.library.pdx.edu/naturalresources_pub Part of the Biodiversity Commons, Biology Commons, and the Zoology Commons Let us know how access to this document benefits ou.y Citation Details Oregon Biodiversity Information Center. 2016. Rare, Threatened and Endangered Species of Oregon. Institute for Natural Resources, Portland State University, Portland, Oregon. 130 pp. This Book is brought to you for free and open access. It has been accepted for inclusion in Institute for Natural Resources Publications by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Authors James S. Kagan, Sue Vrilakas, John A. Christy, Eleanor P. Gaines, Lindsey Wise, Cameron Pahl, and Kathy Howell This book is available at PDXScholar: https://pdxscholar.library.pdx.edu/naturalresources_pub/25 RARE, THREATENED AND ENDANGERED SPECIES OF OREGON OREGON BIODIVERSITY INFORMATION CENTER August 2016 Oregon Biodiversity Information Center Institute for Natural Resources Portland State University P.O. Box 751, -

Habitat Use and Movement Patterns of Two Redwood Forest Salamanders
HABITAT USE AND MOVEMENT PATTERNS OF TWO REDWOOD FOREST SALAMANDERS, ANEIDES VAGRANS AND ENSATINA ESCHSCHOLTZII, WITH AN EXAMINATION OF THE EFFICACY OF PIT TAGS FOR MARKING SMALL PLETHODONTIDS By Christian Brown A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment of the Requirements for the Degree Master of Science in Biology Committee Membership Dr. John Reiss, Committee Chair Dr. Sharyn Marks, Committee Member Dr. Michael Mesler, Committee Member Dr. Stephen Sillett, Committee Member Dr. Erik Jules, Graduate Coordinator May 2017 ABSTRACT HABITAT USE AND MOVEMENT PATTERNS OF TWO REDWOOD FOREST SALAMANDERS, ANEIDES VAGRANS AND ENSATINA ESCHSCHOLTZII, WITH AN EXAMINATION OF THE EFFICACY OF PIT TAGS FOR MARKING SMALL PLETHODONTIDS Christian Brown The habitat use and movements of small, secretive salamanders are generally poorly understood, in part due to the difficulty associated with marking and recapturing such animals. This study was designed to test the efficacy, both in the laboratory and in the field, of using passive integrated transponder (PIT) tags to mark and track two small- bodied plethodontid salamander species native to coastal northwestern California, Aneides vagrans, the Wandering Salamander, and Ensatina eschscholtzii, the Ensatina Salamander. Aneides vagrans inhabits tree crowns. Using cover objects and visual encounter surveys, I searched for A. vagrans in the angiosperm understory canopy at least twice monthly from February 2015 through June 2016. All fieldwork was conducted at the Redwood Experimental Forest, a US Forest Service property in Klamath, California. I found no evidence that A. vagrans is present in this habitat, and thus I could not PIT tag or track the movements of this species. -

Identifying Priority Ecoregions for Amphibian Conservation in the U.S. and Canada
Acknowledgements This assessment was conducted as part of a priority setting effort for Operation Frog Pond, a project of Tree Walkers International. Operation Frog Pond is designed to encourage private individuals and community groups to become involved in amphibian conservation around their homes and communities. Funding for this assessment was provided by The Lawrence Foundation, Northwest Frog Fest, and members of Tree Walkers International. This assessment would not be possible without data provided by The Global Amphibian Assessment, NatureServe, and the International Conservation Union. We are indebted to their foresight in compiling basic scientific information about species’ distributions, ecology, and conservation status; and making these data available to the public, so that we can provide informed stewardship for our natural resources. I would also like to extend a special thank you to Aaron Bloch for compiling conservation status data for amphibians in the United States and to Joe Milmoe and the U.S. Fish and Wildlife Service, Partners for Fish and Wildlife Program for supporting Operation Frog Pond. Photo Credits Photographs are credited to each photographer on the pages where they appear. All rights are reserved by individual photographers. All photos on the front and back cover are copyright Tim Paine. Suggested Citation Brock, B.L. 2007. Identifying priority ecoregions for amphibian conservation in the U.S. and Canada. Tree Walkers International Special Report. Tree Walkers International, USA. Text © 2007 by Brent L. Brock and Tree Walkers International Tree Walkers International, 3025 Woodchuck Road, Bozeman, MT 59715-1702 Layout and design: Elizabeth K. Brock Photographs: as noted, all rights reserved by individual photographers. -
As a Class I Fallen Tree Is Penetrated by the Wood-Boring Beetles and They Begin to Thrive Within It, Nature’S System of Checks and Balances Is Also Activated
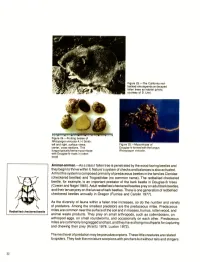
Figure 23.-The California red- backed vole depends on decayed fallen trees as habitat (photo, courtesy of D. Ure). Figure 24.-Fruiting bodies of Rhizopogon vinicolor A. H. Smith: leff and right, surface views; Figure 25.-Mycorrhizae of center, cross sections. This Douglas-fir formed with the fungus fungus typically forms mycorrhizae Rhizopogon vinicolor. with Douglas-fir roots in rotten wood. Animal-animal.-As a class I fallen tree is penetrated by the wood-boring beetles and they begin to thrive within it, Nature’s system of checks and balances is also activated. At first this system is composed primarily of predaceous beetles in the families Cleridae (checkered beetles) and Trogositidae (no common name). The redbellied checkered beetle, for example, is an important predator of the bark beetle in Douglas-fir trees (Cowan and Nagel1965). Adult redbellied checkered beetles prey on adult bark beetles, and their larvae prey on the larvae of bark beetles. There is one generation of redbellied checkered beetles annually in Oregon (Furniss and Carolin 1977). As the diversity of fauna within a fallen tree increases, so do the number and variety of predators. Among the smallest predators are the predaceous mites. Predaceous Redbellied checkered beetle mites are common near the surface of the soil and in mosses, humus, rotten wood, and Janimal waste products. They prey on small arthropods, such as collembolans, on arthropod eggs, on small roundworms, and occasionally on each other. Predaceous mites are commonly long legged and fast, and they have strong mouthparts for capturing and chewing their prey (Krantz 1978, Luxton 1972). -

Wandering Salamander,Aneides Vagrans
COSEWIC Assessment and Status Report on the Wandering Salamander Aneides vagrans in Canada SPECIAL CONCERN 2014 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2014. COSEWIC assessment and status report on the Wandering Salamander Aneides vagrans in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 44 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Production note: COSEWIC would like to acknowledge Barbara Beasley for writing the status report on the Wandering Salamander (Aneides vagrans) in Canada. This report was prepared under contract with Environment Canada and was overseen by Kristiina Ovaska, Co-chair of the COSEWIC Amphibians and Reptiles Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Salamandre errante (Aneides vagrans) au Canada. Cover illustration/photo: Wandering Salamander — Photo taken by Barbara Beasley. Her Majesty the Queen in Right of Canada, 2014. Catalogue No. CW69-14/691-2014E-PDF ISBN 978-1-100-23928-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2014 Common name Wandering Salamander Scientific name Aneides vagrans Status Special Concern Reason for designation The Canadian distribution of this terrestrial salamander is restricted mainly to low elevation forests on Vancouver Island and adjacent small offshore islands in southwestern British Columbia. -

Age Structure and Growth Rates of Two Korean Salamander Species
Animal Cells and Systems Vol. 14, No. 4, December 2010, 315Á322 Age structure and growth rates of two Korean salamander species (Hynobius yangi and Hynobius quelpaertensis) from field populations Jung-Hyun Leea$, Mi-Sook Minb$, Tae-Ho Kimb, Hae-Jun Baekb, Hang Leeb and Daesik Parkc,* aDepartment of Biological Sciences, Kangwon National University, Chuncheon 200-701, Korea; bConservation Genome Resource Bank for Korean Wildlife (CGRB), Research Institute for Veterinary Science, BK21 Program for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea; cDivision of Science Education, Kangwon National University, Chuncheon 200-701, Korea (Received 20 May 2010; received in revised form 23 August 2010; accepted 24 August 2010) We studied and compared the age structure, body size, and growth rates of field populations of two Korean salamander species (Hynobius yangi and Hynobius quelpaertensis) to elucidate important aspects of basic population dynamics of these two endemic Hynobius species. In both populations, females were sexually mature at three years of age, while H. yangi and H. quelpaertensis males matured at two and three years of age, respectively. Both males and females of H. yangi and H. quelpaertensis attained a maximum age of 11 years and 10 years, respectively. In both species, the snout-vent length (SVL) and body weight (BW) of the females were greater than those of the males. The SVL, BW, and asymptotic SVL of both male and female H. yangi were smaller than those of H. quelpaertensis. The adult growth rates after sexual maturation of male and female H. yangi were lower than those of H. -

Level 4-5 Table of Contents
Level 4-5 Table of Contents: 1 Western Bluebirds Live and Work Together 2 Small, Striped and Smelly 3 New Life for a Dead Tree 4 Alligator Lizard Loses His Tail — Or Does He? 5 Often Heard but Seldom Seen: The Western Screech Owl 6 Smallest Hummingbird Flies Long Distance 7 The Dancing, Singing Spotted Towhee Finds His Mate 8 River Pig’s Close Call 9 The Drummer of the Oregon Forest 10 Jumping into the Fire 11 Secretive Giant Salamander Stands its Ground 12 Beaver Kits’ First Adventure Teacher Tracks the Climbing, 13 Skin-Breathing Clouded Salamander 14 The Bobcat Is No Cuddly Kitty Western Bluebirds Live and Work Together Two bright-blue Western bluebirds sat in their home in a woodpecker hole one spring. Around them, young trees were beginning to show bright-green leaves. There were nice, open spaces between the trees for fl ying. The air was warm. Swallows swooped over the river, catching bugs in their beaks. Nuthatches and sparrows fl ew back and forth, eating insects, seeds and berries, and calling to each other. as healthy as the fi rst set. They were all safe and had Western bluebirds are a lot like the other birds that plenty of food. They were never left alone. By the time share their forest. But they do some diff erent and they were big enough to fl y, it was autumn. special things, too. In fall and winter Western bluebirds live in groups. One of these things is that they often have two sets of The young male babysitters stayed in the woodpecker chicks in the same summer.