(Ghb) and Βββ-Hydroxy-Βββ-Methylbutyrate (Hmb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Alcohol Intoxication Withdrawal Adult
Provincial Clinical Knowledge Topic Alcohol Intoxication Withdrawal, Adult Emergency Department V 1.5 © 2018, Alberta Health Services. This work is licensed under the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Disclaimer: This material is intended for use by clinicians only and is provided on an "as is", "where is" basis. Although reasonable efforts were made to confirm the accuracy of the information, Alberta Health Services does not make any representation or warranty, express, implied or statutory, as to the accuracy, reliability, completeness, applicability or fitness for a particular purpose of such information. This material is not a substitute for the advice of a qualified health professional. Alberta Health Services expressly disclaims all liability for the use of these materials, and for any claims, actions, demands or suits arising from such use. Document History Version Date Description of Revision Completed By / Revised By 1.1 July 2015 Completed document (2013) reformatted into Dr. Bullard / Carla new topic template Milligan 1.2 January Minor edits in the Rationale section and form 1 Dr. Bullard / Sarah 2016 info in general care section as well as addition Searle of CIWA-Ar Scoring Reference tool to appendix 1.3 May 2016 Minor edits made to working group Sarah Searle membership list 1.4 June Removed link to Center for Addiction and Dr. Bullard / Sarah 2017 Mental Health assessment and documentation Searle form on pg. 35. Documentation requirements will continue as per local practice at this time. -

Supporting Information
Supporting Information Section 1 Components of DCM based coating strippers Table 1. Composition of Klean Strip Premium. CAS # Components Concentration 75-09-2 Dichloromethane 70.0-95.0% 67-56-1 Methanol < 5.0% 127087-87-0 Poly(oxy-1,2-ethandiyl) < 5.0% 124-38-9 Carbon dioxide < 5.0% Table 2. Composition of Klean Strip X. CAS # Components Concentration 75-09-2 Dichloromethane 30.0 – 40.0% 67-56-1 Methanol 15.0 – 26.0% 67-64-1 Acetone < 10.0% 1330-20-7 Xylene < 10.0% 108-88-3 Toluene < 10.0% 100-41-4 Ethylbenzene < 5.0% 64-17-5 Ethyl alcohol < 5.0% 67-63-0 Isopropyl alcohol < 5.0% Section 2 Sample preparation for the dwell time test As Figure S1 shows, a gasket was pasted on the conformal coating surface and a sheet of parafilm attached to the back of the printed circuit board to avoid solvent leakage dur- ing the dwell test. Figure 1. Sample preparation for the dwell time test. Section 3 Thickness measurement of coated PCBs Materials and Equipment Printed circuit boards (PCB), acrylic conformal coating, tape, Dektak stylus profiler (Bruker, Arizona, USA). Methods A piece of tape was attached on a PCB before coating. The coating was applied on the PCB using the same method as dip coating in the dwell time test. The PCB was sta- tioned and dried at room temperature for over 24 hours. The tape was then torn out to create a coating step. The PCB was fixed on the detection table using tapes. The stylus scanned from sub- strate to coated area. -

The Effects of Ethanol on Ketone Body Metabolism of Fasted Rats Henry S
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1975 The effects of ethanol on ketone body metabolism of fasted rats Henry S. Cabin Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Cabin, Henry S., "The effects of ethanol on ketone body metabolism of fasted rats" (1975). Yale Medicine Thesis Digital Library. 2432. http://elischolar.library.yale.edu/ymtdl/2432 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE MEDICAL LIBRARY YALE MEDICAL LIBRARY Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/effectsofethanolOOcabi The Effects of Ethanol on Ketone Body Metabolism of Fasted Rats by Henry S, Cabin B.A. University of Pennsylvania, 1971 Presented in partial fulfillment of the requirements for the degree of Doctor of Medicine, Yale University School of Medicine -March, 1975- ACKNOWLEDGEMENTS To Dr. Felig- who.has guided me through this research project from its inception, and for whom I have the highest esteem as a teacher, physician and human being. To Rosa, Bill and Andrea- without whose support and assistance this project would never have come to fruition. -

Alcoholic Ketoacidosis
Alcoholic Ketoacidosis: Mind The Gap, Give Them What They Need Brendan Innes BS, Stephanie Carreiro MD University of Massachusetts Medical School, Department of Emergency Medicine Introduction Differential Diagnosis Case Discussion Pancreatitis, Alcohol induced gastritis, Alcohol withdrawal, Diagnostic Criteria for Alcoholic Ketoacidosis2,3 • Patients with alcohol use disorder commonly present to the ED Alcohol induced hepatitis, Acute Kidney Injury, Sepsis, Binge drinking ending in nausea, vomiting, and decreased intake critically ill due to a myriad of underlying pathologies. Metabolic abnormality (Alcoholic ketoacidosis), Acute coronary syndrome, Pulmonary embolism Wide anion gap metabolic acidosis without alternate explanation • Alcoholic ketoacidosis (AKA) should be considered in anyone Clinical Data Positive serum/urine ketones with prolonged and/or binge consumption of alcohol. Low, normal, or slightly elevated serum glucose Anion Gap 36 130 83 27 Urinalysis Core Emergency Medicine Principles • Diagnosis and proper treatment results in rapid correction of 167 Lactate 1.9 5 11 1.9 Protein 2+ • Treatment for AKA requires glucose administration, thiamine underlying metabolic derangements often followed by rapid Salicylate, ethylene glycol, Ketones 3+ supplementation, and volume repletion. methanol not detected Urobilinogen + • D5 NS IV until rehydrated, D5 1/2NS for maintenance. clinical improvement. 16.6 Digoxin: 0.3 ng/mL 17.2 241 RBCs 5/hpf • Thiamine 100 mg IV before glucose. • Failure to make the diagnosis can result in shock, hypokalemia, 49.3 PT/INR: >120/>11 Hyaline casts 21 • Supplement electrolytes PRN. VBG: pH 7.34, pCO2 25 • Continue treatment until anion gap closes, oral intake tolerated. 90% PMNs /hpf hypoglycemia, and acidosis. • Consider other causes of anion gap if gap does not close with Neutrophils 15.6x103/µL BNP: 66 pc/mL UTox: caffeine Trop: 0.1 ng/mL treatment Lipase: 13 U/L Case Description EKG: sinus tach • Consider sodium bicarbonate if despite treatment pH < 7.0. -

Diabetic Ketoacidosis
PRIMER Diabetic ketoacidosis Ketan K. Dhatariya1,2, Nicole S. Glaser3, Ethel Codner4 and Guillermo E. Umpierrez5 ✉ Abstract | Diabetic ketoacidosis (DKA) is the most common acute hyperglycaemic emergency in people with diabetes mellitus. A diagnosis of DKA is confirmed when all of the three criteria are present — ‘D’, either elevated blood glucose levels or a family history of diabetes mellitus; ‘K’, the presence of high urinary or blood ketoacids; and ‘A’, a high anion gap metabolic acidosis. Early diagnosis and management are paramount to improve patient outcomes. The mainstays of treatment include restoration of circulating volume, insulin therapy , electrolyte replacement and treatment of any underlying precipitating event. Without optimal treatment, DKA remains a condition with appreciable, although largely preventable, morbidity and mortality. In this Primer, we discuss the epidemiology , pathogenesis, risk factors and diagnosis of DKA and provide practical recommendations for the management of DKA in adults and children. Circulatory volume Diabetic ketoacidosis (DKA) is the most common acute acid decarboxylase and protein tyrosine phosphatase depletion hyperglycaemic emergency in people with diabetes mel- autoantibodies, as those who present with hyperosmo- A reduction in intravascular litus. DKA is the consequence of an absolute (that is, lar hyperglycaemic state (HHS), and their β-cell func- and/or extracellular fluid total absence of) or relative (that is, levels insufficient tion recovers with restoration of insulin secretion quickly volume, such that there may 2 be an inability to adequately to supress ketone production) lack of insulin and con- after treatment . Thus, individuals with ketosis-prone perfuse tissue. comitant elevation of counter-regulatory hormones, T2DM can often go back to oral glucose-lowering medi- usually resulting in the triad of hyperglycaemia, met- cation without the need for continuing insulin therapy. -

1 Effects of a Ketone-Caffeine Supplement on Cycling And
Effects of A Ketone-Caffeine Supplement On Cycling and Cognitive Performance in Chronic Keto-Adapted Participants THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Madison Lee Bowling Graduate Program in Kinesiology The Ohio State University 2018 Thesis Committee Dr. Jeff Volek Dr. William Kraemer Dr. Carl Maresh 1 Copyrighted by Madison Lee Bowling 2018 2 Abstract As research begins to broaden our understanding of the effects of low carbohydrate, high fat ketogenic diets to different populations, it is crucial to utilize evidence associated with the metabolic and physiological adaptation of chronic implementation. Specific populations are finding that nutritional ketosis may prove advantageous to athletic or cognitive performance. Nutritional ketosis may be identified by an elevated plasma ketone concentration within the blood range 0.5 to 5 mmol/L that results from a chronic implementation of a ketogenic diet. Recently, science shows that ketones contribute to a vast range of therapeutic and performance benefits associated with nutritional ketosis, as a result, exogenous ketone supplements have become commercially available which have proven to induce acute nutritional ketosis without restriction of carbohydrate intake. We previously showed that a supplement containing ketone salts and caffeine significantly increased performance in a non-keto adapted population. To date, there are no reports of whether ketone supplements have an ergogenic effect in an already keto-adapted population. The primary purpose of this study was to determine the performance and metabolic effects of a supplement containing ketone salts and caffeine in a group of people habituated to a ketogenic diet. -

Silfort* SHC1200 Silfort* SHC1200
Technical Data Sheet SilFORT* SHC1200 SilFORT* SHC1200 Description SilFORT SHC1200 Hard Coat SHC1200 hard coat has been found to yield a clear mar-resistant film when applied to a suitably prepared plastic substrate. It can be applied by flow, dip or spray coating. SilFORT SHP401 Primer SHP401 air-dried primer is used as an adhesion promoter for SHC1200 hard coat on polycarbonate resin. It can be applied by flow, dip or spray coating. Key Features and Benefits Fast cure Abrasion resistance Scratch resistance Good clarity Solvent/chemical resistance SHP401 Primer No thermal cure required Improves coating adhesion Improves water resistance Page 1 of 6 *SilFORT ist ein Markenname der Momentive Performance Materials Inc. SilFORT* SHC1200 Improves ultraviolet resistance SHP401 Primer/SHC1200 Hard Coat on polycarbonate (2 - 4 micron Topcoat Thickness) Cured Film Properties Film Thickness, slow dip coat, 10-18 cm/min 2 – 4 micron withdrawal,18-20% solids at 22C Taber Abrasion, 500 cycles 500G on primed < 6.0 % Haze measured per polycarbonate (CS10F wheel) ASTM D1003. * Index of Refraction 1.4 *Humidity during coating and testing will affect final values. Typical Physical Properties Property SHC1200 Hard Coat SHP401 Primer Solids Content, % 20 ± 1 2.1 ± 0.2 Methanol, Isobutanol, 1-Methoxy-2-propanol, Diacetone Solvent Isopropanol Alcohol Flash Point, PMCC 19.4C 36.1C Density, g/cm3 0.911 0.959 pH 7.3 ± 0.2 - Viscosity, cstk @ 25°C 20 ± 3 4 - 7 Dry Film Thickness, 2.75 - 4.5 0.5 micron VOC, g/l 710 937 Patent Status Nothing contained herein shall be construed to imply the nonexistence of any relevant patents or to constitute the permission, inducement or recommendation to practice any invention covered by any patent, without authority from the owner of the patent. -
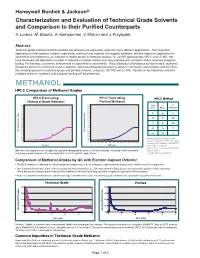
Methanol and Acetone Is in Organic Synthesis
Honeywell Burdick & Jackson® Characterization and Evaluation of Technical Grade Solvents and Comparison to their Purified Counterparts S. Lorenz, M. Bosma, A. Kemperman, V. Mohan and J. Przybytek Abstract: Technical grade solvents and their purified counterparts are separately useful for many different applications. One important application for the common solvents acetonitrile, methanol and acetone is in organic synthesis. Another important application for acetonitrile and methanol is as a diluent or mobile phase in chemical analysis, ie: UV-VIS spectroscopy, HPLC and LC-MS. We have observed and identified a number of impurities in these solvents that may interfere with synthesis and/or accurate analytical testing. For instance, a common contaminant in acetonitrile is acrylonitrile. Trace aldehydes and ketones can be found in methanol. Diacetone alcohol is commonly found in acetone. We have characterized impurity levels in commonly used solvents and will show the variability present in technical grade and purified solvents, using GC, GC-MS and LC-MS. Results on the impurities and their probable effect on synthesis and analytical testing will be presented. METHANOL HPLC Comparison of Methanol Grades HPLC Trace using HPLC Trace using HPLC Method Technical Grade Methanol Purified Methanol 0.200 0.200 Time % % 0.190 0.190 0.180 0.180 (min) Water Methanol 0.170 0.170 0.160 0.160 0.150 0.150 0 95 5 0.140 AU) 0.140 AU) ( ( 0.130 0.130 e e 20 0 100 c 0.120 c 0.120 n 0.110 n 0.110 a a b 0.100 b 0.100 r r 25 0 100 o 0.090 o 0.090 s s 0.080 0.080 -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl -

Alcohol Withdrawal
Alcohol withdrawal TERMINOLOGY CLINICAL CLARIFICATION • Alcohol withdrawal may occur after cessation or reduction of heavy and prolonged alcohol use; manifestations are characterized by autonomic hyperactivity and central nervous system excitation 1, 2 • Severe symptom manifestations (eg, seizures, delirium tremens) may develop in up to 5% of patients 3 CLASSIFICATION • Based on severity ○ Minor alcohol withdrawal syndrome 4, 5 – Manifestations occur early, within the first 48 hours after last drink or decrease in consumption 6 □ Manifestations develop about 6 hours after last drink or decrease in consumption and usually peak about 24 to 36 hours; resolution occurs in 2 to 7 days 7 if withdrawal does not progress to major alcohol withdrawal syndrome 4 – Characterized by mild autonomic hyperactivity (eg, tachycardia, hypertension, diaphoresis, hyperreflexia), mild tremor, anxiety, irritability, sleep disturbances (eg, insomnia, vivid dreams), gastrointestinal symptoms (eg, anorexia, nausea, vomiting), headache, and craving alcohol 4 ○ Major alcohol withdrawal syndrome 5, 4 – Progression and worsening of withdrawal manifestations, usually after about 24 hours from the onset of initial manifestations 4 □ Manifestations often peak around 50 hours before gradual resolution or may continue to progress to severe (complicated) withdrawal, particularly without treatment 4 – Characterized by moderate to severe autonomic hyperactivity (eg, tachycardia, hypertension, diaphoresis, hyperreflexia, fever); marked tremor; pronounced anxiety, insomnia, -

Safety Assessment of Diacetone Alcohol As Used in Cosmetics
Safety Assessment of Diacetone Alcohol as Used in Cosmetics Status: Draft Tentative Report for Panel Review Release Date: February 16, 2021 Panel Meeting Date: March 11 – 12, 2021 The Expert Panel for Cosmetic Ingredient Safety members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; David E. Cohen, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; Lisa A. Peterson, Ph.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. Previous Panel member involved in this assessment: James G. Marks, Jr., M.D. The Cosmetic Ingredient Review (CIR) Executive Director is Bart Heldreth, Ph.D. This safety assessment was prepared by Priya Cherian, Scientific Analyst/Writer, CIR. © Cosmetic Ingredient Review 1620 L Street, NW, Suite 1200 ♢ Washington, DC 20036-4702 ♢ ph 202.331.0651 ♢ fax 202.331.0088 ♢ [email protected] Distributed for Comment Only -- Do Not Cite or Quote Commitment & Credibility since 1976 Memorandum To: Expert Panel for Cosmetic Ingredient Safety Members and Liaisons From: Priya Cherian, Scientific Analyst/Writer, CIR Date: February 16, 2021 Subject: Safety Assessment of Diacetone Alcohol as Used in Cosmetics Enclosed is the Draft Tentative Report of the Safety Assessment of Diacetone Alcohol as Used in Cosmetics (diacet032021rep). At the September 2020 meeting, the Expert Panel for Cosmetic Ingredient Review Safety (Panel) issued an Insufficient Data Announcement (IDA) for this ingredient. In order to come to a conclusion of safety, the Panel requested impurities data. Since the previous review of this report, no new data have been received.