Ep 0095783 A2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Cool Reaction the Endothermic Reaction Between SCIENTIFIC Barium Hydroxide and Ammonium Thiocyanate
Cool Reaction The Endothermic Reaction Between SCIENTIFIC Barium Hydroxide and Ammonium Thiocyanate Introduction Many reactions produce heat, in fact when people think of chemical reactions, heat production is often expected. However, endothermic reactions, reactions which consume heat, can be just as exciting. One of the most striking examples of this is when the solids barium hydroxide and ammonium thiocyanate are mixed together in a beaker. Materials Ammonium thiocyanate, NH4SCN, 10 g Stirring rod Barium hydroxide octahydrate, Ba(OH)28H2O, 20 g Thermometer graduated to at least –30 °C Erlenmeyer flask, small, with stopper, or a 50-mL beaker Safety Precautions Barium salts are toxic by ingestion. Ammonium thiocyanate is also toxic by ingestion. Use caution when handling the beaker or flask. Use tongs if available. The temperatures involved are cold enough to freeze skin. Ammonia vapor is very irritating to eyes and the respiratory tract. Do not allow students to inhale this gas. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Transfer 20 g of barium hydroxide and 10 g of ammonium thiocyanate to a flask and mix with a glass or plastic stirring rod. 2. In less than two minutes the solids become liquid. A thermometer placed in the mixture shows the temperature falling far below freezing. An ammonia odor is evident to all who are near the flask. 3. Place the flask in a small puddle of water and your students will clearly see just how cool this reaction is; the water will freeze the flask to the counter top. -

Writing Total and Net Ionic Equations
WRITING TOTAL AND NET IONIC EQUATIONS http://www.csun.edu/~hcchm001/FreshChemHandouts.html 1. Write the overall equation including the correct designations for the physical state of the substances (s, l, g, aq). Balance this equation. Most of these kinds of equations are double displacement reactions: AX + BY 6 AY + BX 2. For the total ionic equations, write strong electrolytes in solution in the form of aqueous ions. (a) Strong acids. The common strong acids and their aqueous ions are: HI Hydroiodic acid H+-(aq) + I (aq) HBr Hydrobromic acid H+-(aq) + Br (aq) HCl Hydrochloric acid H+-(aq) + Cl (aq) +- HNO33Nitric acid H (aq) + NO (aq) +- HClO44Perchloric acid H (aq) + ClO (aq) +-2 H24SO Sulfuric acid 2 H (aq) + SO4(aq) (b) Strong bases. Strong bases are the hydroxides of the alkali (Group IA) and alkaline earth (Group IIA) metals ions which are sufficiently soluble. The common strong bases and their aqueous ions are: LiOH Lithium hydroxide Li+-(aq) + OH (aq) NaOH Sodium hydroxide Na+-(aq) + OH (aq) KOH Potassium hydroxide K+-(aq) + OH (aq) +2 - Sr(OH)2Strontium hydroxide Sr (aq) + 2 OH (aq) +2 - Ba(OH)2 Barium hydroxide Ba (aq) + 2 OH (aq) (c) Soluble salts. Determinations of the solubility of a salt may be made by reference to SOLUBILITIES OF IONIC COMPOUNDS. Soluble salts are written as their aqueous ions: NaCl(aq) Sodium chloride Na+-(aq) + Cl (aq) +-2 K24SO (aq) Potassium sulfate 2 K (aq) + SO4(aq) +-2 Li23CO (aq) Lithium carbonate 2 Li (aq) + CO3(aq) +-3 Na34PO (aq) Sodium phosphate 3 Na (aq) + PO4(aq) +-2 (NH42) SO4(aq) Ammonium sulfate 2 NH4(aq) + SO4 (aq) 3. -

Acetal (POM) Chemical Compatibility Chart From
ver 31-Mar-2020 Acetal (POM) Chemical Compatibility Chart Chemical Chemical Acetaldehyde A Ammonium Acetate C Acetamide A Ammonium Bifluoride D Acetate Solvents A Ammonium Carbonate D Acetic Acid D Ammonium Caseinate D Acetic Acid, 20% C Ammonium Chloride, 10% B Acetic Acid, 80% D Ammonium Hydroxide D Acetic Acid, Glacial D Ammonium Nitrate, 10% A Acetic Anhydride D Ammonium Oxalate B Acetone A Ammonium Persulfate D Acetyl Chloride, dry D Ammonium Phosphate, Dibasic B Acetylene A Ammonium Phosphate, Monobasic B Alcohols: Amyl A Ammonium Phosphate, Tribasic B Alcohols: Benzyl A Ammonium Sulfate B Alcohols: Butyl A Ammonium Sulfite D Alcohols: Diacetone A Ammonium Thiosulfate B Alcohols: Ethyl A Amyl Acetate B Alcohols: Hexyl A Amyl Alcohol A Alcohols: Isobutyl A Amyl Chloride A Alcohols: Isopropyl A Aniline A Alcohols: Methyl A Aniline Oil D Alcohols: Octyl A Anise Oil D Alcohols: Propyl (1-Propanol) A Antifreeze D Aluminum chloride, 20% C Aqua Regia (80% HCl, 20% HNO3) D Aluminum Fluoride C Aromatic Hydrocarbons A Aluminum Hydroxide A Arsenic Acid D Aluminum Nitrate B Asphalt B Aluminum Potassium Sulfate, 10% C Barium Carbonate A Aluminum Potassium Sulfate, 100% C Barium Chloride A Aluminum Sulfate, 10% B Barium Cyanide B Alums C Barium Hydroxide D Amines D Barium Nitrate B Ammonia, 10% (Ammonium Hydroxide) C Barium Sulfate B Ammonia, 10% D Barium Sulfide A Ammonia, anhydrous D Bay Oil D Ammonia, liquid D Beer A Ammonia Nitrate C Beet Sugar Liquids B Key to General Chemical Resistance – All data is based on ambient or room temperature conditions, about 64°F (18°C) to 73°F (23°C) A = Excellent C = Fair - Moderate Effect, not recommended B= Good - Minor Effect, slight corrosion or discoloration D = Severe Effect, not recommended for ANY use It is the sole responsibility of the system designer and user to select products suitable for their specific application requirements and to ensure proper installation, operation, and maintenance of these products. -

Supporting Information
Supporting Information Section 1 Components of DCM based coating strippers Table 1. Composition of Klean Strip Premium. CAS # Components Concentration 75-09-2 Dichloromethane 70.0-95.0% 67-56-1 Methanol < 5.0% 127087-87-0 Poly(oxy-1,2-ethandiyl) < 5.0% 124-38-9 Carbon dioxide < 5.0% Table 2. Composition of Klean Strip X. CAS # Components Concentration 75-09-2 Dichloromethane 30.0 – 40.0% 67-56-1 Methanol 15.0 – 26.0% 67-64-1 Acetone < 10.0% 1330-20-7 Xylene < 10.0% 108-88-3 Toluene < 10.0% 100-41-4 Ethylbenzene < 5.0% 64-17-5 Ethyl alcohol < 5.0% 67-63-0 Isopropyl alcohol < 5.0% Section 2 Sample preparation for the dwell time test As Figure S1 shows, a gasket was pasted on the conformal coating surface and a sheet of parafilm attached to the back of the printed circuit board to avoid solvent leakage dur- ing the dwell test. Figure 1. Sample preparation for the dwell time test. Section 3 Thickness measurement of coated PCBs Materials and Equipment Printed circuit boards (PCB), acrylic conformal coating, tape, Dektak stylus profiler (Bruker, Arizona, USA). Methods A piece of tape was attached on a PCB before coating. The coating was applied on the PCB using the same method as dip coating in the dwell time test. The PCB was sta- tioned and dried at room temperature for over 24 hours. The tape was then torn out to create a coating step. The PCB was fixed on the detection table using tapes. The stylus scanned from sub- strate to coated area. -

Mccord (Pmccord) – HW6 Acids, Bases and Salts – Mccord – (51520) 1 This Print-Out Should Have 45 Questions
mccord (pmccord) – HW6 Acids, Bases and Salts – mccord – (51520) 1 This print-out should have 45 questions. 6. The term is misleading, because the am- Multiple-choice questions may continue on monium ion is not an acid. the next column or page – find all choices Explanation: before answering. 003 10.0 points 001 10.0 points −9 If the value of Kb for pyridine is 1.8 × 10 , Assume that five weak acids, identified only calculate the equilibrium constant for by numbers (1, 2, 3, 4, and 5), have the + C5H5NH (aq)+ H2O(ℓ) → following ionization constants. + C5H5N(aq) + H3O (aq) . − Ionization 1. 5.6 × 10 6 correct Acid Constant − Ka value 2. 1.8 × 10 9 − 1 1.0 × 10 3 − × −16 2 3.0 × 10 5 3. 1.8 10 − 3 2.6 × 10 7 − × 8 4 4.0 × 10 9 4. 5.6 10 −11 5 7.3 × 10 − 5. −1.8 × 10 9 The anion of which acid is the strongest Explanation: base? 004 10.0 points 1. 4 Which of the following is true in pure water at any temperature? 2. 5 correct 1. Kw decreases with increasing tempera- 3. 2 ture. + − −14 4. 3 2. [H3O ][OH ] = 1.0 × 10 + − 5. 1 3. [H3O ] = [OH ] correct Explanation: 4. pH = 7.0 or greater than 7.0 002 10.0 points 5. pH = 7.0 The term “Ka for the ammonium ion” de- scribes the equilibrium constant for which of Explanation: the following reactions? Kw is shown to INCREASE with increas- ing temperature. pH = 7 is only true when + ⇀ + ◦ + − 1. -

Silfort* SHC1200 Silfort* SHC1200
Technical Data Sheet SilFORT* SHC1200 SilFORT* SHC1200 Description SilFORT SHC1200 Hard Coat SHC1200 hard coat has been found to yield a clear mar-resistant film when applied to a suitably prepared plastic substrate. It can be applied by flow, dip or spray coating. SilFORT SHP401 Primer SHP401 air-dried primer is used as an adhesion promoter for SHC1200 hard coat on polycarbonate resin. It can be applied by flow, dip or spray coating. Key Features and Benefits Fast cure Abrasion resistance Scratch resistance Good clarity Solvent/chemical resistance SHP401 Primer No thermal cure required Improves coating adhesion Improves water resistance Page 1 of 6 *SilFORT ist ein Markenname der Momentive Performance Materials Inc. SilFORT* SHC1200 Improves ultraviolet resistance SHP401 Primer/SHC1200 Hard Coat on polycarbonate (2 - 4 micron Topcoat Thickness) Cured Film Properties Film Thickness, slow dip coat, 10-18 cm/min 2 – 4 micron withdrawal,18-20% solids at 22C Taber Abrasion, 500 cycles 500G on primed < 6.0 % Haze measured per polycarbonate (CS10F wheel) ASTM D1003. * Index of Refraction 1.4 *Humidity during coating and testing will affect final values. Typical Physical Properties Property SHC1200 Hard Coat SHP401 Primer Solids Content, % 20 ± 1 2.1 ± 0.2 Methanol, Isobutanol, 1-Methoxy-2-propanol, Diacetone Solvent Isopropanol Alcohol Flash Point, PMCC 19.4C 36.1C Density, g/cm3 0.911 0.959 pH 7.3 ± 0.2 - Viscosity, cstk @ 25°C 20 ± 3 4 - 7 Dry Film Thickness, 2.75 - 4.5 0.5 micron VOC, g/l 710 937 Patent Status Nothing contained herein shall be construed to imply the nonexistence of any relevant patents or to constitute the permission, inducement or recommendation to practice any invention covered by any patent, without authority from the owner of the patent. -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12 -
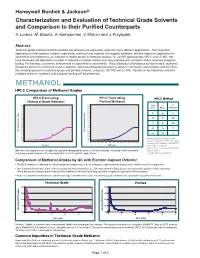
Methanol and Acetone Is in Organic Synthesis
Honeywell Burdick & Jackson® Characterization and Evaluation of Technical Grade Solvents and Comparison to their Purified Counterparts S. Lorenz, M. Bosma, A. Kemperman, V. Mohan and J. Przybytek Abstract: Technical grade solvents and their purified counterparts are separately useful for many different applications. One important application for the common solvents acetonitrile, methanol and acetone is in organic synthesis. Another important application for acetonitrile and methanol is as a diluent or mobile phase in chemical analysis, ie: UV-VIS spectroscopy, HPLC and LC-MS. We have observed and identified a number of impurities in these solvents that may interfere with synthesis and/or accurate analytical testing. For instance, a common contaminant in acetonitrile is acrylonitrile. Trace aldehydes and ketones can be found in methanol. Diacetone alcohol is commonly found in acetone. We have characterized impurity levels in commonly used solvents and will show the variability present in technical grade and purified solvents, using GC, GC-MS and LC-MS. Results on the impurities and their probable effect on synthesis and analytical testing will be presented. METHANOL HPLC Comparison of Methanol Grades HPLC Trace using HPLC Trace using HPLC Method Technical Grade Methanol Purified Methanol 0.200 0.200 Time % % 0.190 0.190 0.180 0.180 (min) Water Methanol 0.170 0.170 0.160 0.160 0.150 0.150 0 95 5 0.140 AU) 0.140 AU) ( ( 0.130 0.130 e e 20 0 100 c 0.120 c 0.120 n 0.110 n 0.110 a a b 0.100 b 0.100 r r 25 0 100 o 0.090 o 0.090 s s 0.080 0.080 -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl -

Toxicological Profile for Barium and Barium Compounds
TOXICOLOGICAL PROFILE FOR BARIUM AND BARIUM COMPOUNDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 BARIUM AND BARIUM COMPOUNDS ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. BARIUM AND BARIUM COMPOUNDS iii UPDATE STATEMENT A Toxicological Profile for Barium and Barium Compounds, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 BARIUM AND BARIUM COMPOUNDS iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 26-Sep-2009 Revision Date 10-Feb-2021 Revision Number 2 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Barium hydroxide, anhydrous Cat No. : 12195 CAS-No 17194-00-2 EC-No. 241-234-5 Molecular Formula BaH2O2 Reach Registration Number - 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Based on available data, the classification criteria are not met Health hazards ______________________________________________________________________________________________ ALFAA12195 Page 1 / 11 SAFETY DATA SHEET Barium hydroxide, anhydrous Revision Date 10-Feb-2021 ______________________________________________________________________________________________ Acute oral toxicity Category 4 (H302) Acute Inhalation Toxicity - Dusts and Mists