Why Are Passerine Eggshells Spotted? Using Calcium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PDF 10/20/08 Loro Parque, Tenerife Vogel Park (Walsrode Birdpark)
News Highlights • News Highlights • News Highlights • News Highlights • News Highlights • News Highlights had another round of breeding, where all the Loro Parque, Tenerife eggs were fertile. Two eggs were damaged as May 2008 before and also got some help with glue on the egg-shell. However they died too. Th e other two eggs developed very well and are now to- gether with their adoptive parents. Th is pair of Green-winged Macaws (Ara chloroptera) will rear the chicks aft er their hatching. Th is experienced older breeding pair, as well as rearing its own chicks, last year raised two Buff on’s Macaws (Ara ambigua), these chicks being green, and the year before red! Th is year The Bewick’s Swan (Cygnus colombianus bewicki) the babies are expected to be coloured blue. incubated and hatched four young. Usually, small pink featherless macaw chicks Recently hatched Pesquet’s Parrot (Psittrichas Th e Bewick’s Swan ( fulgidus) all look almost the same. Th e parent-off spring Cygnus colombianus relationship of these birds is normally so bewicki) incubated and hatched four young. Now the breeding season is approaching strong, that if the chicks later develop another Th is was good news as most of the other Swan its climax. Over 400 youngsters have been al- colour, the parents do not have any problems species in the Park has been unsuccessful this ready ringed, and a lot of eggs have been laid. with the rearing. All of the adoptions have season. Th is year we would again like to present some been successful Nest-controls revealed that the colony of highlights about this. -

West New Britain
WEST NEW BRITAIN SEPTEMBER 7–14, 2019 Superb Fruit-Dove LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM WEST NEW BRITAIN SEPTEMBER 7–14, 2019 By Dion Hobcroft Who is a pretty girl: two male Eclectus Parrots vie for the attention of the red female. This parrot has an unusual reproductive biology where females stay at the nest hollow and mate with several males. Victor Emanuel Nature Tours 2 West New Britain, 2019 Planes took off and landed on time, customs and immigration was pretty speedy, and we were soon checked into our lovely rooms at the Airways Hotel. Everything went to plan, so we were well set up to spend the afternoon at Pacific Adventist University, a pleasant campus with several freshwater ponds and patches of scrubby secondary growth dominated by large Rain Trees that are native to central America. It is a great location to start a trip, as the open terrain and good numbers of water birds make birding easy, and there are quite a number of species that we typically only encounter at this location. These we found with little effort including a fine Orange-fronted Fruit-Dove, several Plumed Whistling- Ducks, excellent Radjah Shelducks, a nesting Comb-crested Jacana, nesting Papuan Frogmouth, and great views of Black-backed Butcherbird and the dapper little Gray-headed Munia. A highlight was studying the elaborate bower of the Fawn-breasted Bowerbird. Other species seen well included Wandering Whistling- Duck, Common Kingfisher, Bar-shouldered Dove, Whistling Kite, Metallic Starling, and Australasian Figbird. -
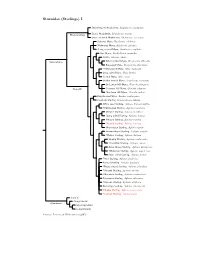
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

West New Britain Extension July 22–27, 2017
WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 White-mantled Kingfisher (Dion Hobcroft) LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM WEST NEW BRITAIN EXTENSION JULY 22 –27, 2017 By Dion Hobcroft A Purple-bellied Lory dines out in a Coconut flower. (Dion Hobcroft) Our tour got off to a shaky start with a cancelled flight from Port Moresby to Hoskins. Luckily, the superb Airways Hotel honored our rooms from the previous day that we had missed due to another cancelled flight. Love you Air Niugini! After settling in to the very comfortable Walindi Dive Resort, with its well-planned rooms, tasty meals, and excellent staff, we headed out for our first birding in New Britain. We met Joel, a local villager, who guided us up beyond his village into the forest. He led us to a New Britain Boobook, a small hawk-owl he keeps tabs on (and has done so for the past few years). With the elections on, I joked with Joel that we should call the owl “Prime Minister Pete.” We had actually seen the Prime Minister the day before. Victor Emanuel Nature Tours 2 West New Britain Extension, 2017 After great looks at “Pete” we slowly wandered to a viewing area over the forest that was heaving with birds —lots of Eclectus Parrots, our first Blue-eyed Cockatoos, dozens of Red-knobbed and Yellowish imperial-pigeons, Long-tailed Myna, Variable Goshawk, an all-white Pied Coucal, a single Channel-billed Cuckoo (quite rare on NB), the beautiful Purple-bellied Lory, the scarce Black-bellied Myzomela, and great views of the colorful Knob-billed Fruit-Dove. -

In the Market for Extinction: an Inventory of Jakarta's Bird Markets
TRAFFIC IN THE MARKET FOR EXTINCTION REPORT An inventory of Jakarta’s bird markets Serene C.L. Chng, James A. Eaton, Kanitha Krishnasamy, Chris R. Shepherd SEPTEMBER 2015 and Vincent Nijman TRAFFIC Report: In the Market i for Extinction: An inventory of Jakarta’s bird markets i TRAFFIC REPORT TRAFFIC, the wild life trade monitoring net work, which is the leading non-governmental organization working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. TRAFFIC is a strategic alliance of WWF and IUCN . Reprod uction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations con cern ing the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views of the authors expressed in this publication are those of the writers and do not necessarily reflect those of TRAFFIC, WWF or IUCN. Published by TRAFFIC. Southeast Asia Regional Office Unit 3-2, 1st Floor, Jalan SS23/11 Taman SEA, 47400 Petaling Jaya Selangor, Malaysia Telephone : (603) 7880 3940 Fax : (603) 7882 0171 Copyright of material published in this report is vested in TRAFFIC © TRAFFIC 2015. ISBN 978-983-3393 UK Registered Charity No. 1076722. Suggested citation: Chng, S.C.L., Eaton, J.A., Krishnasamy, K., Shepherd, C.R. and Nijman, V. (2015) In the Market for Extinction: An inventory of Jakarta’s bird markets. -

Papua New Guinea Highlights July 26–August 8, 2018
PAPUA NEW GUINEA HIGHLIGHTS JULY 26–AUGUST 8, 2018 Ribbon-tailed Astrapia © Dion Hobcroft LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM The amazing Southern Crowned-Pigeon on a nest at Kiunga. (Terry Cloudman) Papua New Guinea offers some of the most exciting birding on the planet. On this adventure, combined with West New Britain, we recorded some 338 species. A perfect storm of events preceded our tour—the largest earthquake in living memory in the Southern Highlands, a contested election result, and the with- holding of mining royalties as clan lineages are sorted out—caused much local upset. At the last minute, like all other tour companies, we changed the itinerary to stay at Rondon Ridge in the Central Highlands rather than Ambua Lodge in Hela Province. I thought this might adversely affect our bird list but was pleasantly surprised when we came in right on what we observed last year. Here is an account of our tour. Arriving on a Saturday afternoon with everything going to plan, we found ourselves birding in the afternoon along the roadside at Brown River, some 50 kilometers from Port Moresby. This is lowland forest at this location, and we Victor Emanuel Nature Tours 2 Papua New Guinea Highlights, 2018 racked up some 40 species in our 90 minutes here. These included great views of staple lowland fare like Orange-bellied and Pink-spotted fruit-doves, flyover Zoe’s and Pinon imperial-pigeons, Coconut and Yellow-streaked lories, and good numbers of Metallic Starlings that are always in a hurry commuting over the forest. -
Prehistoric Birds from New Ireland, Papua New Guinea: Extinctions on a Large Melanesian Island
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 2563–2568, March 1999 Anthropology, Evolution Prehistoric birds from New Ireland, Papua New Guinea: Extinctions on a large Melanesian island DAVID W. STEADMAN*†,J.PETER WHITE‡, AND JIM ALLEN§ *Florida Museum of Natural History, University of Florida, P.O. Box 117800, Gainesville, FL 32611; ‡School of Archaeology, University of Sydney, Sydney, New South Wales 2006, Australia; and §Department of Archaeology, La Trobe University, Bundoora, Victoria 3083, Australia Communicated by Patrick V. Kirch, University of California, Berkeley, CA, December 11, 1998 (received for review September 10, 1998) ABSTRACT At least 50 species of birds are represented in the avifauna of New Ireland has changed in species composi- 241 bird bones from five late Pleistocene and Holocene tion since the first arrival of humans. archaeological sites on New Ireland (Bismarck Archipelago, Papua New Guinea). The bones include only two of seabirds MATERIALS AND METHODS and none of migrant shorebirds or introduced species. Of the 50 species, at least 12 (petrel, hawk, megapode, quail, four The prehistoric bones (summarized in Table 1) belong to the rails, cockatoo, two owls, and crow) are not part of the current Papua New Guinea National Museum and Art Gallery (Port avifauna and have not been recorded previously from New Moresby, Papua New Guinea). We believe that most of the Ireland. Larger samples of bones undoubtedly would indicate bird bones were accumulated by prehistoric peoples rather more extirpated species and refine the chronology of extinc- than non-human predators because of the clearly cultural tion. Humans have lived on New Ireland for ca. -

Late Pleistocene Songbirds of Liang Bua (Flores
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2017 Late Pleistocene songbirds of Liang Bua (Flores, Indonesia); the first fossil passerine fauna described from Wallacea Hanneke J.M Meijer Smithsonian National Museum of Natural History, Naturalis Biodiversity Center, University of Bergen, [email protected] Rokus Awe Due Pusat Penelitian Arkeologi Nasional Thomas Sutikna University of Wollongong, [email protected] Wahyu Saptomo Pusat Penelitian Arkeologi Nasional - Jatmiko National Research and Development Centre for Archaeology, Indonesia, Pusat Arkeologi Nasional, University of Wollongong See next page for additional authors Publication Details Meijer, H. J.M., Awe Due, R., Sutikna, T., Saptomo, W., Jatmiko, , Wasisto, S., Tocheri, M. W. & Mayr, G. (2017). Late Pleistocene songbirds of Liang Bua (Flores, Indonesia); the first fossil passerine fauna described from Wallacea. PeerJ, 5 e3676-1-e3676-25. Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Late Pleistocene songbirds of Liang Bua (Flores, Indonesia); the first fossil passerine fauna described from Wallacea Abstract Background Passerines (Aves: Passeriformes) dominate modern terrestrial bird communities yet their fossil record is limited. Liang Bua is a large cave on the Indonesian island of Flores that preserves Late Pleistocene- Holocene deposits (∼190 ka to present day). Birds are the most diverse faunal group at Liang Bua and are present throughout the stratigraphic sequence. Methods We examined avian remains from the Late Pleistocene deposits of Sector XII, a 2 x 2 m area excavated to about 8.5 m depth. -
Species List
Sunrise Birding, LLC http://www.sunrisebirding.com PAPUA NEW GUINEA June 2009 – SPECIES LIST Leaders: Steve Bird, Gina Nichol, and local guides Ne = near endemic E = Endemic SPECIES Scientific Name Days out Highest daily of 14 seen count: L/C= Locally Common h = Heard only 1. Little Grebe Tachybaptus ruficollis 1 3 2. Little Pied Cormorant Phalacrocorax melanoleucos 5 6 3. Little Black Cormorant Phalacrocorax melanoleucos 3 50 4. Lesser Frigatebird Fregata ariel 1 1 5. Great Egret Egretta alba 4 2 6. Eastern Reef Egret Egretta sacra 2 4 7. Rufous Night-Heron Nycticorax caledonicus 3 3 8. Intermediate Egret Ardea intermedia 2 3 9. Great-billed Heron Ardea sumatrana 2 2 10. Pied Heron Ardea picata 2 40 11. Cattle Egret Ardea ibis 5 L/C 12. Black Bittern Ixobrychus flavicollis 1 1 13. Australian Ibis Threskiornis molucca 1 1 14. Spotted Whistling-Duck Dendrocygna guttata Ne 1 1 15. Wandering Whistling-Duck Dendrocygna arcuata 1 30 16. Plumed Whistling-Duck Dendrocygna eytoni 1 1 17. Pacific Black Duck Anas superciliosa 4 20 18. Osprey Pandion haliaetus 3 2 19. Crested Hawk (Pacific Baza) Aviceda subcristata 1 2 20. Long-tailed Honey Buzzard Henicopernis longicauda 2 3 21. Black-winged Kite Elanus caeruleus 2 1 22. Black Kite Milvus migrans 7 10+ 23. Whistling Kite Haliastur sphenurus 6 6+ 24. Brahminy Kite Haliastur indus 10 6+ 25. White-bellied Sea-Eagle Haliaeetus leucogaster 3 2 26. Variable (Grey) Goshawk Accipiter hiogaster 5 2 27. New Guinea Harpy-Eagle Harpyopsis novaeguineae E 1 1 V 28. Gurney's Eagle Aquila gurneyi Ne 1 1 29. -

New Or Confirmatory Information on Some Species of New Guinean Birds
VOL. 10 (7) SEPTEMBER 1984 209 New or Confirmatory Information on Some Species of New Guinean Birds By H. L. BELL, Department of Zoology, University of New England, Armidale, N.S.W. 2351 Summary Information is presented on Netta pus coromandelianus (extension of range); Aviceda subcristata (herbivory); Harpyopsis novaeguineae (hunting); Falco severus (attacking waders); Tumix maculosa (tree roosting); Eulabeomis plumbeiventris (extension of range, habitat, breeding, identification); Rein wardtoena reinwardtsi (ground feeding); Chalcophaps indica (speed of flight); C stephani (nesting); Henicophaps albifrons (nesting, calls, flushing); Galli columba rufigula (foraging on display grounds of Diphyllodes, calls); Trugon terrestris (flushing, calls, attraction to running water); Ptilinopus nanus (nectarivory); Ducula pinon (display); Chalcopsitta scintilla fa (drinking fermented liquids, melanism and/or affinities with C atra, feeding of fledglings, size of parties); Neopsittacus musschenbroekii and N pullicauda (comparison of behaviours); Micropsitta pusio (eating termites); Opopsitta gulielmitertii (eating hard seeds); Scythrops novaehollandiae (breeding status); Ptilorrhoa caerulescens (nesting); Rhipidura threnothorax (foraging); Monarcha melanopsis (possible breeding in New Guinea); M chrysomela (display, nesting); Arses telescophlhalmus (appeasement display); Pachycephala leucogaster (extension of range, affinities with P. monacha); Aplonis metallica (nectarivory, nesting with raptor); Mino anais (extension of range, nesting). Introduction -

Papua New Guinea Highlights September 12–25, 2019
PAPUA NEW GUINEA HIGHLIGHTS SEPTEMBER 12–25, 2019 Raggiana Bird of Paradise LEADER: DION HOBCROFT LIST COMPILED BY: DION HOBCROFT VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM PAPUA NEW GUINEA HIGHLIGHTS SEPTEMBER 12–25, 2019 By Dion Hobcroft The extraordinary male King of Saxony Bird of Paradise has occipital plumes more than twice as long as the bird itself. They can independently twirl the plumes. Victor Emanuel Nature Tours 2 Papua New Guinea Highlights, 2019 After our successful pre-trip to New Britain, we returned to Port Moresby. With everything going to plan with the new inbound participants, there was time to organize an afternoon trip to the village of Lea Lea on the coast south of Port Moresby. We made stops at mangrove mudflats, small freshwater ponds, patches of tropical Eucalyptus woodland and, finally, right on the coast in a patch of coconuts and scrubby village vegetation. The mudflats produced Australian Gull- billed Terns hawking for crabs; the ponds turned up a lovely Buff-banded Rail; and the enormous Blue-winged Kookaburra was a big hit with participants. Despite stiff winds we quickly had good views of our two major targets on the coast—the localized Silver-eared Honeyeater and Varied Honeyeater. Villagers were selling fish, betel nut, and fruit and vegetables. It was a public holiday—Independence Day—and many people were dressed up and the mood festive. This male Raggiana Bird of Paradise was wonderfully tame this year in Varirata National Park. Victor Emanuel Nature Tours 3 Papua New Guinea Highlights, 2019 The day in Varirata National Park, one of my favorite birding locations on the planet, is a big day of the tour. -

A Comprehensive Molecular Phylogeny Of
Molecular Phylogenetics and Evolution 44 (2007) 1031–1056 www.elsevier.com/locate/ympev A comprehensive molecular phylogeny of the starlings (Aves: Sturnidae) and mockingbirds (Aves: Mimidae): Congruent mtDNA and nuclear trees for a cosmopolitan avian radiation Irby J. Lovette a,*, Dustin R. Rubenstein a,b,1 a Fuller Evolutionary Biology Program, Laboratory of Ornithology, Cornell University, Ithaca, NY 14950, USA b Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14850, USA Received 27 September 2006; revised 15 March 2007; accepted 19 March 2007 Available online 5 April 2007 Abstract We generated a comprehensive phylogeny for the avian families Sturnidae (starlings, mynas, Rhabdornis, oxpeckers, and allies) and Mimidae (mockingbirds, thrashers, and allies) to explore patterns of morphological and behavioral diversification. Reconstructions were based on mitochondrial DNA sequences from five coding genes (4108 bp), and nuclear intron sequences from four loci (2974 bp), for most taxa, supplemented with NDII gene sequences (1041 bp) derived from museum skin specimens from additional taxa; together the 117 sampled taxa comprise 78% of the 151 species in these families and include representatives of all currently or recently recognized genera. Phylogenetic analyses consistently identified nine major clades. The basal lineage is comprised of the two Buphagus oxpeckers, which are presently confined to Africa where they are obligately associated with large mammals. Some species in nearly all of the other major clades also feed on or around large vertebrates, and this association may be an ancestral trait that fostered the world-wide dis- persal of this group. The remaining taxa divide into sister clades representing the New-World Mimidae and Old-World Sturnidae.