System Design and Experimental Study of a 80K/10W Pulse Tube Cooler Driven by a Linear Motor Compressor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

Measuring the Steady State Thermal Efficiency of a Heating System
Measuring the Steady State Thermal Efficiency of a Heating System. Last week you had a tour of “Colby Power and Light” and had a chance to see Gus Libby’s really cool, campus-scale, multi-million dollar calorimeter. This week we will use lab-scale calorimeters to measure the heat and carbon content of different fuels (methane, propane, butane, and gasoline). Our simple calorimeters were designed by Whitney King and Chuck Jones, Science Division Instrument Wizard, to simulate the highly efficient HTP Versa Flame fire-tube boiler. The lab has three parts: 1) Calibrate the calorimeter 2) Measure the heat content of different fuels 3) Compare the measured heat content to the calculated heat content 1) Calibrate the Calorimeter. You lab instructor will demonstrate the assembly of the laboratory- scale fire-tube calorimeter. The heat transferred from the burning fuel to the calorimeter can be determined from the mass of the object being heated, the heat capacity of the object and the change in temperature. � = ���� ∗ �� ∗ ∆� We will begin the experiment by adding a known amount of heat to our calorimeters from a coffee cup heater. The heater is a simple resistor that converts electrical energy into heat. The heater has a resistance of 47.5 ohms and is plugged into a 120 V outlet. Recalling your electricity basics, we know that V=IR where V is the voltage in Figure 1. HTP Versa Flame Fire Tube volts, I is the current in amps, and R is the Boiler. Brown Tubes carry the hot resistance in ohms. The power, P, consumed by combustion gasses inside of the the heater is volts times current, water tank where they condense releasing the maximum available 2 heat. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -
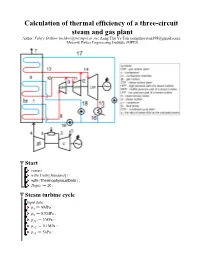
Calculation of Thermal Efficiency of a Three-Circuit Steam and Gas Plant
Calculation of thermal efficiency of a three-circuit steam and gas plant Author: Valery Ochkov ([email protected]), Aung Thu Ya Tun ([email protected]), Moscow Power Engineering Institute (MPEI) Start > > > > Steam turbine cycle Input data: > > > > > > > > > > > > > > Functions and procedures The specific enthalpy of the steam entering the turbine HPP: > (2.1) The specific enthalpy of steam at outlet of the turbine HPP: > (2.2) Specific work of HPP steam turbine: > (2.3) The temperature of the steam at the outlet of the HPP turbine: > (2.4) The specific enthalpy of steam at the point 10: > (2.5) > (2.6) The balance of the mixing streams at points 9 and 10: > (2.7) The temperature of steam at the inlet to the MPP of the turbine: > (2.8) > (2.8) > (2.9) > Specific enthalpy of water vapor at the inlet to the MPP of the turbine: > (2.10) Specific enthalpy of steam at the outlet of the turbine MPP: > (2.11) C : > (2.12) The temperature of the steam at the outlet of the MPP of the turbine: > (2.13) The specific enthalpy of steam at point 13: > (2.14) > (2.15) Balance when mixing streams at points 12 and 13: > (2.16) The temperature of steam at the inlet to the LPP of the turbine: > (2.17) > (2.18) The specific enthalpy of steam at outlet of the turbine LPP: > (2.19) Specific LPP operation of a steam turbine: > (2.8) > (2.20) The degree of dryness of the steam at the outlet of the turbine LPP: > (2.21) Condensate temperature: > (2.22) Specific enthalpy of condensate: > (2.23) The specific enthalpy of the feed water high pressure -

Numerical Analysis of the 4 K Regenerator in a Pulse Tube Cryocooler
263 Numerical Analysis of the 4 K Regenerator in a Pulse Tube Cryocooler Y.Zhao1,2, W.Dai1, Y.Chen1, X.Wang1, E.Luo1 1Key Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190 China 2University of Chinese Academy of Sciences, Beijing 100049, China ABSTRACT The numerical simulation of a 4 K pulse tube cryocooler is difficult due to its complexity of nature. The main difficulty comes from the oscillatory flow combined with the real gas effects. In this paper, the numerical simulation of the 4 K regenerator in a pulse tube cryocooler with a relatively high frequency has been performed with the commercial CFD software, FLUENT [1]. FLUENT takes into account the non-ideal gas properties of 4He, the properties of the magnetic regenerative material, and the heat transfer inside the heat exchanger and regenerator. Interesting features such as the acoustic work flow, temperature distribution, the volume flow, etc., have been systematically studied. A cooling power of 0.36 W was obtained numerically at the temperature of 4.2 K. The study takes a deeper look into the working mechanism of a 4 K pulse tube cryocooler as well as setting the basis for optimizing a pulse tube cooler driven by a Vuillemier type thermal compressor. INTRODUCTION Cryocoolers working at liquid helium temperatures could be used in the application fields of space exploration, military, medical and low-temperature physics. Especially, for many applications, a 4 K pulse tube cryocooler is a potential candidate because of the merits of no cryogenic moving components. Owing to the advances in cooler design and the development of magnetic regenerative materials [2-4], a cooling temperature below 4 K was first obtained with a three-stage Gifford McMahon (G-M) type pulse tube refrigerator [5]. -

Methodology for Thermal Efficiency and Energy Input Calculations and Analysis of Biomass Cogeneration Unit Characteristics
Technical Support Document for the Revisions to Definition of Cogeneration Unit in Clean Air Interstate Rule (CAIR), CAIR Federal Implementation Plan, Clean Air Mercury Rule (CAMR), and CAMR Proposed Federal Plan; Revision to National Emission Standards for Hazardous Air Pollutants for Industrial, Commercial, and Institutional Boilers and Process Heaters; and Technical Corrections to CAIR and Acid Rain Program Rules Methodology for Thermal Efficiency and Energy Input Calculations and Analysis of Biomass Cogeneration Unit Characteristics EPA Docket number: EPA-HQ-OAR-2007-0012 April 2007 U.S. Environmental Protection Agency Office of Air and Radiation Technical Support Document for Proposed Revisions to Cogeneration Definition in CAIR, CAIR FIP, CAMR, and Proposed CAMR Federal Plan This Technical Support Document (TSD) has several purposes. One purpose of the TSD is to set forth the methodology for determining the thermal efficiency of a unit for purposes of applying the definition of the term “cogeneration unit” under the existing CAIR, the CAIR model trading rules, the CAIR FIP, CAMR, the CAMR Hg model trading rule, and the proposed CAMR Federal Plan. Another purpose of the TSD is to present information relevant to the proposed revisions, and other potential revisions for which EPA is requesting comment, concerning the thermal efficiency standard. One of the critical values used in the determination of thermal efficiency is the “total energy input” of the unit. Consequently, in connection with setting forth the methodology for determining thermal efficiency, the TSD specifically addresses what formula is to be used in calculating a unit’s total energy input under the existing rules. There are two major issues concerning the calculation of total energy input. -

The Use of Geothermal Energy in Absorption Refrigeration Circuits
TECHNICAL TRANSACTIONS CZASOPISMO TECHNICZNE ENVIRONMENT ENGINEERING ŚRODOWISKO 1-Ś/2015 RENATA SIKORSKA-BĄCZEK* THE USE OF GEOTHERMAL ENERGY IN ABSORPTION REFRIGERATION CIRCUITS ZASTOSOWANIE ENERGII GEOTERMALNEJ DO NAPĘDU ZIĘBIAREK ABSORPCYJNYCH Abstract The use of absorption refrigerators to produce cold can definitely reduce energy usage through the use of a heat from the geothermal resources. Absorption refrigerators do not have a negative impact on the natural environment. They are particularly economically attractive when there is a free energy source with a temperature of between 50 and 200°C. This article presents a hybrid absorption-compression refrigerator powered by geothermal energy used for the production of ice slurry for air-conditioning refrigeration. Keywords: hybrid absorption/compression refrigerator, water-ammonia absorption refri- gerator, geothermal energy Streszczenie Zastosowanie ziębiarek absorpcyjnych do produkcji zimna pozwala zdecydowanie ograni- czyć straty energii dzięki wykorzystaniu do ich napędu ciepła pochodzącego ze złóż geotermal- nych. Ziębiarki absorpcyjne nie mają negatywnego wpływu na środowisko naturalne. Są one szczególnie atrakcyjne ekonomicznie gdy istnieje darmowe źródło energii o temperaturze 50‒200°C. W artykule przedstawiono ziębiarkę hybrydową absorpcyjno-sprężarkową zasilaną energią geotermalną wykorzystywaną do produkcji lodu zawiesinowego do celów ziębienia w klimatyzacji. Słowa kluczowe: ziębiarka hybrydowa absorpcyjno/sprężarkowa, ziębiarka absorpcyjna wodno-amoniakalna, energia geotermalna DOI: 10.4467/2353737XCT.15.190.4395 * Ph.D. Eng. Renata Sikorska-Bączek, Institute of Thermal Engineering and Air Protection, Faculty of Environmental Engineering, Cracow University of Technology. 120 1. Introduction Due to the dwindling global resources of fossil fuels, and also because of the need to protect the environment from degradation, methods using alternative energy sources are becoming more and more popular. -
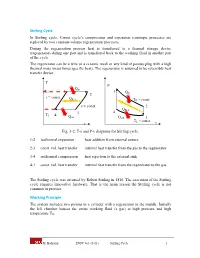
Stirling Cycle Working Principle
Stirling Cycle In Stirling cycle, Carnot cycle’s compression and expansion isentropic processes are replaced by two constant-volume regeneration processes. During the regeneration process heat is transferred to a thermal storage device (regenerator) during one part and is transferred back to the working fluid in another part of the cycle. The regenerator can be a wire or a ceramic mesh or any kind of porous plug with a high thermal mass (mass times specific heat). The regenerator is assumed to be reversible heat transfer device. T P Qin TH 1 1 Q 2 in v = const. Q Reg. TH = const. v = const. 2 QReg. 3 4 TL 4 Qout Qout 3 T = const. s L v Fig. 3-2: T-s and P-v diagrams for Stirling cycle. 1-2 isothermal expansion heat addition from external source 2-3 const. vol. heat transfer internal heat transfer from the gas to the regenerator 3-4 isothermal compression heat rejection to the external sink 4-1 const. vol. heat transfer internal heat transfer from the regenerator to the gas The Stirling cycle was invented by Robert Stirling in 1816. The execution of the Stirling cycle requires innovative hardware. That is the main reason the Stirling cycle is not common in practice. Working Principle The system includes two pistons in a cylinder with a regenerator in the middle. Initially the left chamber houses the entire working fluid (a gas) at high pressure and high temperature TH. M. Bahrami ENSC 461 (S 11) Stirling Cycle 1 TH TL QH State 1 Regenerator Fig 3-3a: 1-2, isothermal heat transfer to the gas at TH from external source. -

Removing the Mystery of Entropy and Thermodynamics – Part IV Harvey S
Removing the Mystery of Entropy and Thermodynamics – Part IV Harvey S. Leff, Reed College, Portland, ORa,b In Part IV of this five-part series,1–3 reversibility and irreversibility are discussed. The question-answer format of Part I is continued and Key Points 4.1–4.3 are enumerated.. Questions and Answers T < TA. That energy enters through a surface, heats the matter • What is a reversible process? Recall that a near that surface to a temperature greater than T, and subse- reversible process is specified in the Clausius algorithm, quently energy spreads to other parts of the system at lower dS = đQrev /T. To appreciate this subtlety, it is important to un- temperature. The system’s temperature is nonuniform during derstand the significance of reversible processes in thermody- the ensuing energy-spreading process, nonequilibrium ther- namics. Although they are idealized processes that can only be modynamic states are reached, and the process is irreversible. approximated in real life, they are extremely useful. A revers- To approximate reversible heating, say, at constant pres- ible process is typically infinitely slow and sequential, based on sure, one can put a system in contact with many successively a large number of small steps that can be reversed in principle. hotter reservoirs. In Fig. 1 this idea is illustrated using only In the limit of an infinite number of vanishingly small steps, all initial, final, and three intermediate reservoirs, each separated thermodynamic states encountered for all subsystems and sur- by a finite temperature change. Step 1 takes the system from roundings are equilibrium states, and the process is reversible. -

Chapter 5 the Second Law of Thermodynamics
Chapter 5 The Second Law of Thermodynamics 5.1 Statements of The Second Law Consider a power cycle shown in Figure 5.1-1a that has the following characteristics. The power cycle absorbs 1000 kJ of heat from a high temperature heat source and performs 1200 kJ of work. Applying the first law to this system we obtain, ∆Ecycle = Qcycle + Wcycle 0 = 1000 + (− 1200) = − 200 kJ This is obviously impossible and a clear violation of the first law of thermodyanmics as 200 kJ of energy have been created. High T High T Qcycle = 1000 kJ Qcycle = 1000 kJ System System Wcycle = 1200 kJ Wcycle = 1000 kJ (a) Violation of the first law (b) Obey the first law Figure 5.1-1 (a) A power cycle receiving 1000 kJ of heat and performing 1200 kJ of work, which is a violation of the law of conservation of energy. (b) A power cycle receiving 1000 kJ of heat and performing 1000 kJ of work, which does not violate the law of conservation of energy. Consider next the power cycle shown in Figure 5.1-1b where the system absorbs 1000 kJ of heat from a high temperature heat source and performs 10200 kJ of work. Applying the first law to this system we obtain, ∆Ecycle = Qcycle + Wcycle 0 = 1000 + (− 1000) = 0 The first law of thermodynamics is satisfied, and all would seem to be well. However, it is a fact that no one has ever succeeded in creating a device that will produce 1000 kJ of work from 1000 kJ of heat. -
Quantum Mechanics Thermal Efficiency
Quantum Mechanics_thermal efficiency In Thermodynamics, the thermal efficiency ( ) is a dimensionless performance measure of a device that uses thermal energy, such as aninternal combustion engine, a steam turbine or asteam engine, a boiler, a furnace, or arefrigerator for example. In other words, efficiency indicates how well an energy conversion or transfer process is accomplished. Overview Output (mechanical) energy is always lower than input energy In general, energy conversion efficiency is the ratio between the useful output of adevice and the input, in energy terms. For thermal efficiency, the input, , to the device is Heat, or the heat-content of a fuel that is consumed. The desired output is mechanical Work, , or heat, , or possibly both. Because the input heat normally has a real financial cost, a memorable, generic definition of thermal efficiency is[1] From the first law of thermodynamics, the energy output cannot exceed the input, so When expressed as a percentage, the thermal efficiency must be between 0% and 100%. Efficiency is typically less than 100% because there are inefficiencies such as friction and heat loss that convert the energy into alternative forms. For example, a typical gasoline automobile engine operates at around 25% efficiency, and a large coal- fueled electrical generating plant peaks at about 46%. The largest diesel engine in the world peaks at 51.7%. In a combined cycle plant, thermal efficiencies are approaching 60%.[2] Such a real-world value may be used as a figure of meritfor the device. For engines where a fuel is burned there are two types of thermal efficiency: indicated thermal efficiency and brake thermal efficiency.[3] This efficiency is only appropriate when comparing similar types or similar devices. -

7-122 a Solar Pond Power Plant Operates by Absorbing Heat from the Hot Region Near the Bottom, and Rejecting Waste Heat to the Cold Region Near the Top
7-49 7-122 A solar pond power plant operates by absorbing heat from the hot region near the bottom, and rejecting waste heat to the cold region near the top. The maximum thermal efficiency that the power plant can have is to be determined. Analysis The highest thermal efficiency a heat engine operating between two specified temperature limits can have is the Carnot efficiency, which is determined from TL 308 K 12.7% 80 C th,max th,C 1 1 0.127 or TH 353 K In reality, the temperature of the working fluid must be above 35 C HE in the condenser, and below 80 C in the boiler to allow for any W effective heat transfer. Therefore, the maximum efficiency of the actual heat engine will be lower than the value calculated above. 35 C 7-123 A Carnot heat engine cycle is executed in a closed system with a fixed mass of steam. The net work output of the cycle and the ratio of sink and source temperatures are given. The low temperature in the cycle is to be determined. Assumptions The engine is said to operate on the Carnot cycle, which is totally reversible. Analysis The thermal efficiency of the cycle is TL 1 th 1 1 0.5 Carnot HE TH 2 Also, W W 25kJ Q 50kJ th H QH th 0.5 QL QH W 50 25 25 kJ and 0.0103 kg QL 25 kJ q 2427.2 kJ/kg h H2O L m 0.0103 kg fg@TL since the enthalpy of vaporization hfg at a given T or P represents the amount of heat transfer as 1 kg of a substance is converted from saturated liquid to saturated vapor at that T or P.