Studies on Environmental Relevance of Quorum Sensing Signal Decay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Classification of Esterases: an Important Gene Family Involved in Insecticide Resistance - a Review
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 107(4): 437-449, June 2012 437 The classification of esterases: an important gene family involved in insecticide resistance - A Review Isabela Reis Montella1,2, Renata Schama1,2,3/+, Denise Valle1,2,3 1Laboratório de Fisiologia e Controle de Artrópodes Vetores, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21040-900 Rio de Janeiro, RJ, Brasil 2Instituto de Biologia do Exército, Rio de Janeiro, RJ, Brasil 3Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular, Rio de Janeiro, RJ, Brasil The use of chemical insecticides continues to play a major role in the control of disease vector populations, which is leading to the global dissemination of insecticide resistance. A greater capacity to detoxify insecticides, due to an increase in the expression or activity of three major enzyme families, also known as metabolic resistance, is one major resistance mechanisms. The esterase family of enzymes hydrolyse ester bonds, which are present in a wide range of insecticides; therefore, these enzymes may be involved in resistance to the main chemicals employed in control programs. Historically, insecticide resistance has driven research on insect esterases and schemes for their classification. Currently, several different nomenclatures are used to describe the esterases of distinct species and a universal standard classification does not exist. The esterase gene family appears to be rapidly evolving and each insect species has a unique complement of detoxification genes with only a few orthologues across species. The examples listed in this review cover different aspects of their biochemical nature. However, they do not appear to contribute to reliably distinguish among the different resistance mechanisms. -

The Gene for Albicidin Detoxification from Pantoea Dispersa Encodes An
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 9984–9989, September 1997 Plant Biology The gene for albicidin detoxification from Pantoea dispersa encodes an esterase and attenuates pathogenicity of Xanthomonas albilineans to sugarcane (phytotoxin resistanceyleaf scald diseaseyserine hydrolaseypathogenicity factor) LIANHUI ZHANG* AND ROBERT G. BIRCH Department of Botany, The University of Queensland, Brisbane 4072, Australia Communicated by Allen Kerr, University of Adelaide, Adelaide, Australia, June 16, 1997 (received for review April 4, 1997) ABSTRACT Albicidin phytotoxins are pathogenicity fac- produces a family of antibiotics and phytotoxins that block tors in a devastating disease of sugarcane known as leaf scald, DNA replication in bacteria and sugarcane proplastids (13, caused by Xanthomonas albilineans. A gene (albD) from Pantoea 14). The major toxin, named albicidin, has been partially dispersa has been cloned and sequenced and been shown to characterized as a low Mr compound with several aromatic code for a peptide of 235 amino acids that detoxifies albicidin. rings. Because albicidin is rapidly bactericidal to a range of The gene shows no significant homology at the DNA or protein Gram-positive and Gram-negative bacteria at concentrations level to any known sequence, but the gene product contains a as low as 1 ng ml21, it is also of interest as a potential clinical GxSxG motif that is conserved in serine hydrolases. The AlbD antibiotic (15). protein, purified to homogeneity by means of a glutathione Symptoms of leaf scald disease include the emergence of S-transferase gene fusion system, showed strong esterase chlorotic leaves, wilting, necrosis, and sometimes rapid death activity on p-nitrophenyl butyrate and released hydrophilic of plants, often after a prolonged latent period. -

Protein Network Analyses of Pulmonary Endothelial Cells In
www.nature.com/scientificreports OPEN Protein network analyses of pulmonary endothelial cells in chronic thromboembolic pulmonary hypertension Sarath Babu Nukala1,8,9*, Olga Tura‑Ceide3,4,5,9, Giancarlo Aldini1, Valérie F. E. D. Smolders2,3, Isabel Blanco3,4, Victor I. Peinado3,4, Manuel Castell6, Joan Albert Barber3,4, Alessandra Altomare1, Giovanna Baron1, Marina Carini1, Marta Cascante2,7,9 & Alfonsina D’Amato1,9* Chronic thromboembolic pulmonary hypertension (CTEPH) is a vascular disease characterized by the presence of organized thromboembolic material in pulmonary arteries leading to increased vascular resistance, heart failure and death. Dysfunction of endothelial cells is involved in CTEPH. The present study describes for the frst time the molecular processes underlying endothelial dysfunction in the development of the CTEPH. The advanced analytical approach and the protein network analyses of patient derived CTEPH endothelial cells allowed the quantitation of 3258 proteins. The 673 diferentially regulated proteins were associated with functional and disease protein network modules. The protein network analyses resulted in the characterization of dysregulated pathways associated with endothelial dysfunction, such as mitochondrial dysfunction, oxidative phosphorylation, sirtuin signaling, infammatory response, oxidative stress and fatty acid metabolism related pathways. In addition, the quantifcation of advanced oxidation protein products, total protein carbonyl content, and intracellular reactive oxygen species resulted increased -

Glycolytic Strategy As a Tradeoff Between Energy Yield and Protein Cost
Glycolytic strategy as a tradeoff between energy SEE COMMENTARY yield and protein cost Avi Flamholza,1, Elad Noora,1, Arren Bar-Evena, Wolfram Liebermeistera,b, and Ron Miloa,2 aDepartment of Plant Sciences, The Weizmann Institute of Science, Rehovot 76100, Israel; and bInstitut für Biochemie, Charité–Universitätsmedizin Berlin, 10117 Berlin, Germany Edited by Richard E. Lenski, Michigan State University, East Lansing, MI, and approved April 4, 2013 (received for review September 17, 2012) Contrary to the textbook portrayal of glycolysis as a single pathway sequence from glyceraldehyde 3-phosphate (G3P) through pyruvate conserved across all domains of life, not all sugar-consuming known as “lower glycolysis.” In the EMP pathway, glucose is organisms use the canonical Embden–Meyerhoff–Parnass (EMP) phosphorylated twice and cleaved into two triose-phosphates glycolytic pathway. Prokaryotic glucose metabolism is particularly (G3P and dihydroxyacetone phosphate), both of which are used to diverse, including several alternative glycolytic pathways, the most produce ATP through substrate-level phosphorylation in lower gly- common of which is the Entner–Doudoroff (ED) pathway. The prev- colysis (2, 7) (Fig. 1B). In the ED pathway, glucose is phosphory- alence of the ED pathway is puzzling as it produces only one ATP lated only once and oxidized to 2-keto-3-deoxy-6-phosphogluconate per glucose—half as much as the EMP pathway. We argue that the (KDPG), which is cleaved into one pyruvate and one G3P. Pyruvate diversity of prokaryotic glucose metabolism may reflect a tradeoff does not support substrate-level phosphorylation (7) and so, in between a pathway’s energy (ATP) yield and the amount of enzy- the ED pathway, only one of the cleavage products (G3P) is used matic protein required to catalyze pathway flux. -

Reevaluation of Serum Arylesterase Activity in Neurodevelopmental Disorders
antioxidants Article Reevaluation of Serum Arylesterase Activity in Neurodevelopmental Disorders Ignazio Stefano Piras 1,2, Stefano Gabriele 1, Laura Altieri 1, Federica Lombardi 1, Roberto Sacco 1, Carla Lintas 1 , Barbara Manzi 3, Paolo Curatolo 3, Maria Nobile 4, Catia Rigoletto 4, Massimo Molteni 4 and Antonio M. Persico 5,* 1 Unit of Child & Adolescent Neuropsychiatry, University Campus Bio-Medico, I-00128 Rome, Italy; [email protected] (I.S.P.); [email protected] (S.G.); [email protected] (L.A.); [email protected] (F.L.); [email protected] (R.S.); [email protected] (C.L.) 2 Neurogenomics Division, The Translational Genomics Research Institute, Phoenix, AZ 85254, USA 3 Unit of Child and Adolescent Neuropsychiatry, University of Rome “Tor Vergata”, I-00133 Rome, Italy; [email protected] (B.M.); [email protected] (P.C.) 4 Child Psychopathology Unit, Scientific Institute, IRCCS ‘E. Medea’, I-23842 Bosisio Parini (LC), Italy; [email protected] (M.N.); [email protected] (C.R.); [email protected] (M.M.) 5 Interdepartmental Program “Autism 0–90”, “G. Martino” University Hospital, University of Messina, I-98122 Messina, Italy * Correspondence: [email protected] Abstract: Organophosphate compounds (OPs) interfere with neurodevelopment and are neuro- toxic for humans and animals. They are first biotransformed to the more toxic oxon form, and then hydrolyzed to specific metabolites by the enzyme paraoxonase/arylesterase, encoded by the gene PON1 located on human chr. 7q21.3. In autism spectrum disorder (ASD) and in attention- deficit/hyperactivity disorder (ADHD), a correlation between OP exposure and disease onset has Citation: Piras, I.S.; Gabriele, S.; been reported. -

Chapter 23 Gluconeogenesis Gluconeogenesis, Con't
BCH 4054 Fall 2000 Chapter 23 Lecture Notes Slide 1 Chapter 23 Gluconeogenesis Glycogen Metabolism Pentose Phosphate Pathway Slide 2 Gluconeogenesis • Humans use about 160 g of glucose per day, about 75% for the brain. • Body fluids and glycogen stores supply only a little over a day’s supply. • In absence of dietary carbohydrate, the needed glucose must be made from non- carbohydrate precursors. • That process is called gluconeogenesis. Slide 3 Gluconeogenesis, con’t. • Brain and muscle consume most of the glucose. • Liver and kidney are the main sites of gluconeogenesis. • Substrates include pyruvate, lactate, glycerol, most amino acids, and all TCA intermediates. • Fatty acids cannot be converted to glucose in animals. • (They can in plants because of the glyoxalate cycle.) Chapter 23, page 1 Slide 4 Remember it is necessary for the pathways to differ in some respects, Gluconeogenesis, con’t. so that the overall G can be negative in each direction. Usually • Substrates include anything that can be converted the steps with large negative G of to phosphoenolpyruvate . one pathway are replaced in the • Many of the reactions are the same as those in reverse pathway with reactions that glycolysis. have a large negative G in the • All glycolytic reactions which are near equilibrium can opposite direction. operate in both directions. • The three glycolytic reactions far from equilibrium (large -DG) must be bypassed. • A side by side comparison is shown in Fig 23.1. Slide 5 Unique Reactions of Gluconeogenesis • Recall that pyruvate kinase, though named in reverse, is not reversible and has a DG of –23 kJ/mol. -

Arylesterase Phenotype-Specific Positive Association Between Arylesterase Activity and Cholinesterase Specific Activity in Human Serum
Int. J. Environ. Res. Public Health 2014, 11, 1422-1443; doi:10.3390/ijerph110201422 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Article Arylesterase Phenotype-Specific Positive Association Between Arylesterase Activity and Cholinesterase Specific Activity in Human Serum 1, 2,3 1 Yutaka Aoki *, Kathy J. Helzlsouer and Paul T. Strickland 1 Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA; E-Mail: [email protected] 2 Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA 3 Mercy Medical Center, Baltimore, MD 21202, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +1-410-404-9153. Received: 3 June 2013; in revised form: 27 December 2013 / Accepted: 15 January 2014 / Published: 27 January 2014 Abstract: Context: Cholinesterase (ChE) specific activity is the ratio of ChE activity to ChE mass and, as a biomarker of exposure to cholinesterase inhibitors, has a potential advantage over simple ChE activity. Objective: To examine the association of several potential correlates (serum arylesterase/paraoxonase activity, serum albumin, sex, age, month of blood collection, and smoking) with plasma ChE specific activity. Methods: We analyzed data from 195 cancer-free controls from a nested case-control study, accounting for potential confounding. Results: Arylesterase activity had an independent, statistically significant positive association with ChE specific activity, and its magnitude was the greatest for the arylesterase phenotype corresponding to the QQ PON1192 genotype followed by phenotypes corresponding to QR and RR genotypes. -

WO 2013/016115 Al 31 January 2013 (31.01.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/016115 Al 31 January 2013 (31.01.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every CUP 19/14 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US20 12/047326 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (22) International Filing Date: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, 19 July 2012 (19.07.2012) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/5 10,637 22 July 201 1 (22.07.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US) : NO- GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, VOZYMES NORTH AMERICA, INC. -

Thermostable Enzymes Important for Industrial Biotechnology
Thermostable Enzymes Important For Industrial Biotechnology Submitted by Aaron Charles Westlake to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological Science in September 2018 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Aaron Charles Westlake [1] Abstract The use of enzymes in technology is of increasing commercial interest due to their high catalytic efficiency and specificity and the lowering of manufacturing costs. Enzymes are also becoming more widely utilised because they are more environmentally friendly compared to chemical methods. Firstly, they carry out their reactions at ambient temperatures requiring less energy to achieve the high temperatures and pressures that many chemical methods require. Secondly, they can substitute for toxic chemical catalysts which need careful disposal. In this project two classes of enzymes of industrial interest from thermophiles were investigated, lactonase enzymes and 1-deoxy-D-xylulose 5-phosphate (DXP) synthases. A quorum sensing lactonase from Vulcanisaeta moutnovskia, a thermoacidophilic anaerobic crenarchaeon, was expressed in high levels in an Escherichia coli host, then purified and characterised with a range of industrially relevant substrates. These enzymes are of industrial interest for water treatment and bioreactors for their ability to prevent biofilm formation in bacteria. This enzyme showed different specificity to another well characterised quorum sensing lactonase from a thermophilic crenarchaeon, Sulfolobus solfataricus. -

Serum Paraoxonase and Arylesterase Activities in Metabolic Syndrome In
European Journal of Endocrinology (2011) 164 219–222 ISSN 0804-4643 CLINICAL STUDY Serum paraoxonase and arylesterase activities in metabolic syndrome in Zahedan, southeast Iran Mohammad Hashemi, Dor Mohammad Kordi-Tamandani1, Nooshin Sharifi1, Abdolkarim Moazeni-Roodi2, Mahmoud-Ali Kaykhaei3, Behzad Narouie3 and Adam Torkmanzehi1 Department of Clinical Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan 98167-43175, Iran, 1Department of Biology, Faculty of Sciences, University of Sistan and Baluchestan, Zahedan 98155-987, Iran and 2Research Center for Infectious Diseases and Tropical Medicine and 3Department of Internal Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan 98167-43175, Iran (Correspondence should be addressed to M Hashemi; Email: [email protected]) Abstract Objective: Paraoxonase (PON) is associated with high-density lipoprotein and protects serum lipid from oxidation. The aim of this study was to determine serum PON, arylesterase (ARE) activities, and total antioxidant capacity (TAC) in metabolic syndrome (MES). Methods: This case–control study was performed on 106 patients with MES and 231 healthy subjects. Serum PON and ARE activities were determined spectrophotometrically. TAC was determined using ferric reducing ability of plasma assay. Results: The results showed that serum PON activity was significantly lower in patients with MES (69.62G59.86 IU/l) than healthy subjects (91.64G77.45 IU/l) (P!0.05). The serum ARE activity in MES and normal subjects were 45.23G23.24 and 65.69G31.10 kU/l respectively. The ARE activity was significantly lower in patients with MES than normal subjects (P!0.0001). No significant differences were observed between MES and normal subjects regarding TAC. -

O O2 Enzymes Available from Sigma Enzymes Available from Sigma
COO 2.7.1.15 Ribokinase OXIDOREDUCTASES CONH2 COO 2.7.1.16 Ribulokinase 1.1.1.1 Alcohol dehydrogenase BLOOD GROUP + O O + O O 1.1.1.3 Homoserine dehydrogenase HYALURONIC ACID DERMATAN ALGINATES O-ANTIGENS STARCH GLYCOGEN CH COO N COO 2.7.1.17 Xylulokinase P GLYCOPROTEINS SUBSTANCES 2 OH N + COO 1.1.1.8 Glycerol-3-phosphate dehydrogenase Ribose -O - P - O - P - O- Adenosine(P) Ribose - O - P - O - P - O -Adenosine NICOTINATE 2.7.1.19 Phosphoribulokinase GANGLIOSIDES PEPTIDO- CH OH CH OH N 1 + COO 1.1.1.9 D-Xylulose reductase 2 2 NH .2.1 2.7.1.24 Dephospho-CoA kinase O CHITIN CHONDROITIN PECTIN INULIN CELLULOSE O O NH O O O O Ribose- P 2.4 N N RP 1.1.1.10 l-Xylulose reductase MUCINS GLYCAN 6.3.5.1 2.7.7.18 2.7.1.25 Adenylylsulfate kinase CH2OH HO Indoleacetate Indoxyl + 1.1.1.14 l-Iditol dehydrogenase L O O O Desamino-NAD Nicotinate- Quinolinate- A 2.7.1.28 Triokinase O O 1.1.1.132 HO (Auxin) NAD(P) 6.3.1.5 2.4.2.19 1.1.1.19 Glucuronate reductase CHOH - 2.4.1.68 CH3 OH OH OH nucleotide 2.7.1.30 Glycerol kinase Y - COO nucleotide 2.7.1.31 Glycerate kinase 1.1.1.21 Aldehyde reductase AcNH CHOH COO 6.3.2.7-10 2.4.1.69 O 1.2.3.7 2.4.2.19 R OPPT OH OH + 1.1.1.22 UDPglucose dehydrogenase 2.4.99.7 HO O OPPU HO 2.7.1.32 Choline kinase S CH2OH 6.3.2.13 OH OPPU CH HO CH2CH(NH3)COO HO CH CH NH HO CH2CH2NHCOCH3 CH O CH CH NHCOCH COO 1.1.1.23 Histidinol dehydrogenase OPC 2.4.1.17 3 2.4.1.29 CH CHO 2 2 2 3 2 2 3 O 2.7.1.33 Pantothenate kinase CH3CH NHAC OH OH OH LACTOSE 2 COO 1.1.1.25 Shikimate dehydrogenase A HO HO OPPG CH OH 2.7.1.34 Pantetheine kinase UDP- TDP-Rhamnose 2 NH NH NH NH N M 2.7.1.36 Mevalonate kinase 1.1.1.27 Lactate dehydrogenase HO COO- GDP- 2.4.1.21 O NH NH 4.1.1.28 2.3.1.5 2.1.1.4 1.1.1.29 Glycerate dehydrogenase C UDP-N-Ac-Muramate Iduronate OH 2.4.1.1 2.4.1.11 HO 5-Hydroxy- 5-Hydroxytryptamine N-Acetyl-serotonin N-Acetyl-5-O-methyl-serotonin Quinolinate 2.7.1.39 Homoserine kinase Mannuronate CH3 etc. -
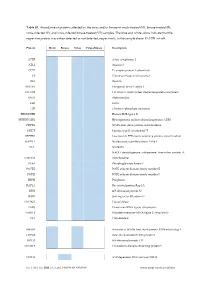
Virus-Infected (V), and Virus-Infected Binase-Treated (VB) Samples
Table S1. Abundance of proteins affected by the virus and/or binase in mock-treated (M), binase-treated (B), virus-infected (V), and virus-infected binase-treated (VB) samples. The blue and white colors indicate that the respective protein was either detected or not detected, respectively, in the sample above 1% FDR cut-off. Protein Mock Binase Virus Virus+Binase Description ACTB Actin, cytoplasmic 1 ATL2 Atlastin-2 CCT7 T-complex protein 1 subunit eta CS Citrate synthase, mitochondrial DES Desmin EEF1A1 Elongation factor 1-alpha 1 EFTUD2 116 kDa U5 small nuclear ribonucleoprotein component ENO1 Alpha-enolase EZR Ezrin GPI Glucose-6-phosphate isomerase HIST1H2BB Histone H2B type 1-B HNRNPA2B1 Heterogeneous nuclear ribonucleoproteins A2/B1 HSPD1 60 kDa heat shock protein, mitochondrial KRT75 Keratin, type II cytoskeletal 75 LRPPRC Leucine-rich PPR motif-containing protein, mitochondrial NAP1L1 Nucleosome assembly protein 1-like 1 NCL Nucleolin NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, NDUFS8 mitochondrial PGK1 Phosphoglycerate kinase 1 POTEE POTE ankyrin domain family member E POTEI POTE ankyrin domain family member I PRPH Peripherin RAP1A Ras-related protein Rap-1A RPS2 40S ribosomal protein S2 SF3B1 Splicing factor 3B subunit 1 TALDO1 Transaldolase TARS Threonine--tRNA ligase, cytoplasmic TARSL2 Probable threonine--tRNA ligase 2, cytoplasmic TKT Transketolase AHSA1 Activator of 90 kDa heat shock protein ATPase homolog 1 HSPA2 Heat shock-related 70 kDa protein 2 RPL15 60S ribosomal protein L15 TXNDC5 Thioredoxin domain-containing protein 5 DDX18 ATP-dependent RNA helicase DDX18 Int. J. Mol. Sci. 2020, 21, x; doi: FOR PEER REVIEW www.mdpi.com/journal/ijms Int.