Modified Polysaccharides, Method for Their Production and Their Use
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complementary and Alternative Treatments for Alopecia: a Comprehensive Review
Review Article Skin Appendage Disord 2019;5:72–89 Received: April 22, 2018 DOI: 10.1159/000492035 Accepted: July 10, 2018 Published online: August 21, 2018 Complementary and Alternative Treatments for Alopecia: A Comprehensive Review Anna-Marie Hosking Margit Juhasz Natasha Atanaskova Mesinkovska Department of Dermatology, University of California, Irvine, Irvine, CA, USA Keywords Introduction Alopecia · Complementary and alternative medicine · Efficacy According to the National Center for Complementary and Integrative Health (NCCIH), a branch of the Nation- al Institutes of Health (NIH; Bethesda, MD, USA), more Abstract than 30% of adults and 12% of children utilize treatments The treatment of alopecia is limited by a lack of therapies developed “outside of mainstream Western, or conven- that induce and sustain disease remission. Given the nega- tional, medicine,” with a total USD 30.2 billion out-of- tive psychosocial impact of hair loss, patients that do not pocket dollars spent annually [1]. In the treatment of alo- see significant hair restoration with conventional therapies pecia, there is an unmet need for therapies providing sat- often turn to complementary and alternative medicine isfying, long-term results. Patients often turn to (CAM). Although there are a variety of CAM treatment op- complementary and alternative medicine (CAM) in an tions on the market for alopecia, only a few are backed by attempt to find safe, natural, and efficacious therapies to multiple randomized controlled trials. Further, these mo- restore hair. Although CAMs boast hair-growing poten- dalities are not regulated by the Food and Drug Administra- tial, patients may be disappointed with results as there is tion and there is a lack of standardization of bioactive in- a lack of standardization of bioactive ingredients and lim- gredients in over-the-counter vitamins, herbs, and supple- ited scientific evidence. -

WO 2014/167554 A2 16 October 2014 (16.10.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/167554 A2 16 October 2014 (16.10.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/IB20 14/060675 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 12 April 2014 (12.04.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: zw. 1103/DEL/2013 12 April 2013 (12.04.2013) IN (84) Designated States (unless otherwise indicated, for every (71) Applicant: VYOME BIOSCIENCES PVT. LTD. kind of regional protection available): ARIPO (BW, GH, [IN/IN]; 459 F.I.E, First Floor, Patparganj Industrial Area, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, New Delhi 110092 (IN). -

What's the Best Treatment for Cradle Cap?
From the CLINIcAL InQUiRiES Family Physicians Inquiries Network Ryan C. Sheffield, MD, Paul Crawford, MD What’s the best treatment Eglin Air Force Base Family Medicine Residency, Eglin Air for cradle cap? Force Base, Fla Sarah Towner Wright, MLS University of North Carolina at Chapel Hill Evidence-based answer Ketoconazole (Nizoral) shampoo appears corticosteroids to severe cases because to be a safe and efficacious treatment of possible systemic absorption (SOR: C). for infants with cradle cap (strength of Overnight application of emollients followed recommendation [SOR]: C, consensus, by gentle brushing and washing with usual practice, opinion, disease-oriented baby shampoo helps to remove the scale evidence, and case series). Limit topical associated with cradle cap (SOR: C). ® Dowden Health Media Clinical commentary ICopyrightf parents can’t leave it be, recommend brush to loosen the scale. Although mineral oil andFor a brush personal to loosen scale use noonly evidence supports this, it seems safe Cradle cap is distressing to parents. They and is somewhat effective. want everyone else to see how gorgeous This review makes me feel more FAST TRACK their new baby is, and cradle cap can make comfortable with recommending ketocon- their beautiful little one look scruffy. My azole shampoo when mineral oil proves If parents need standard therapy has been to stress to the insufficient. For resistant cases, a cute hat to do something, parents that it isn’t a problem for the baby. can work wonders. If the parents still want to do something -

Table 1B 2005 Community Prescription Numbers, Together with Government
TABLE 1B 2005 COMMUNITY PRESCRIPTION NUMBERS, TOGETHER WITH GOVERNMENT AND PATIENT COSTS FOR PBS-LISTED DRUGS Table 1B includes an estimate of community (non-public hospital) prescription numbers for the 2005 calendar year, costs (government and patient contribution) for the items with a four digit PBS/RPBS code, together with the defined daily dose (DDD) where assigned. There is no cost information available for items with a five digit Amfac drug code. Table 1 exclude the presentation of information on any item with an estimated community use of less than 110 prescriptions in 2005. The prescription items are arranged by ATC code on generic name, and by form and strength using either the PBS drug code (4 digit) or, for non-PBS items, the Amfac drug code (5 digit). Consult the index (page 255) by generic drug name to obtain the appropriate ATC code. An index by 2nd level of the ATC classification follows: ALIMENTARY TRACT AND METABOLISM PAGE NO A01 STOMATOLOGICAL PREPARATIONS 181 A02 DRUGS FOR ACID RELATED DISORDERS 182 A03 DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS 185 A04 ANTIEMETICS AND ANTINAUSEANTS 187 A05 BILE AND LIVER THERAPY 188 A06 LAXATIVES 189 A07 ANTIDIARRHOEALS, INTESTINAL 191 ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS A08 ANTIOBESITY PREPARATIONS, EXCLUDING DIET PRODUCTS 193 A09 DIGESTIVES, INCLUDING ENZYMES 194 A10 DRUGS USED IN DIABETES 195 A11 VITAMINS 197 A12 MINERAL SUPPLEMENTS 199 A14 ANABOLIC AGENTS FOR SYSTEMIC USE 200 177 BLOOD AND BLOOD FORMING ORGANS B01 ANTITHROMBOTIC AGENTS 201 B02 ANTIHAEMORRHAGICS 203 B03 -

Zinc Therapy in Dermatology: a Review
Hindawi Publishing Corporation Dermatology Research and Practice Volume 2014, Article ID 709152, 11 pages http://dx.doi.org/10.1155/2014/709152 Review Article Zinc Therapy in Dermatology: A Review Mrinal Gupta, Vikram K. Mahajan, Karaninder S. Mehta, and Pushpinder S. Chauhan DepartmentofDermatology,Venereology&Leprosy,Dr.R.P.Govt.MedicalCollege,Kangra(Tanda),HimachalPradesh176001,India Correspondence should be addressed to Vikram K. Mahajan; [email protected] Received 1 May 2014; Accepted 23 June 2014; Published 10 July 2014 Academic Editor: Craig G. Burkhart Copyright © 2014 Mrinal Gupta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Zinc, both in elemental or in its salt forms, has been used as a therapeutic modality for centuries. Topical preparations like zinc oxide, calamine, or zinc pyrithione have been in use as photoprotecting, soothing agents or as active ingredient of antidandruff shampoos. Its use has expanded manifold over the years for a number of dermatological conditions including infections (leishmaniasis, warts), inflammatory dermatoses (acne vulgaris, rosacea), pigmentary disorders (melasma), and neoplasias (basal cell carcinoma). Although the role of oral zinc is well-established in human zinc deficiency syndromes including acrodermatitis enteropathica, it is only in recent years that importance of zinc as a micronutrient essential for infant -

Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services
Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services Australian Statistics on Medicines 1997 i © Commonwealth of Australia 1998 ISBN 0 642 36772 8 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be repoduced by any process without written permission from AusInfo. Requests and enquiries concerning reproduction and rights should be directed to the Manager, Legislative Services, AusInfo, GPO Box 1920, Canberra, ACT 2601. Publication approval number 2446 ii FOREWORD The Australian Statistics on Medicines (ASM) is an annual publication produced by the Drug Utilisation Sub-Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee. Comprehensive drug utilisation data are required for a number of purposes including pharmacosurveillance and the targeting and evaluation of quality use of medicines initiatives. It is also needed by regulatory and financing authorities and by the Pharmaceutical Industry. A major aim of the ASM has been to put comprehensive and valid statistics on the Australian use of medicines in the public domain to allow access by all interested parties. Publication of the Australian data facilitates international comparisons of drug utilisation profiles, and encourages international collaboration on drug utilisation research particularly in relation to enhancing the quality use of medicines and health outcomes. The data available in the ASM represent estimates of the aggregate community use (non public hospital) of prescription medicines in Australia. In 1997 the estimated number of prescriptions dispensed through community pharmacies was 179 million prescriptions, a level of increase over 1996 of only 0.4% which was less than the increase in population (1.2%). -

(12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding Et Al
US 20140051633A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding et al. (43) Pub. Date: Feb. 20, 2014 (54) HEPATOCYTE GROWTH FACTOR MIMICS (60) Provisional application No. 61/706,567, filed on Sep. AS THERAPEUTICAGENTS 27, 2012, provisional application No. 61/706,437, filed on Sep. 27, 2012. (71) Applicant: Washington State University, Pullman, WA (US) Publication Classification (72) Inventors: Joseph W. Harding, Pullman, WA (US); John W. Wright, Pullman, WA (US); (51) Int. C. Caroline C. Benoist, Nashville, TN C07K5/065 (2006.01) (US); Leen H. Kawas, Pullman, WA (52) U.S. C. (US); Gary A. Wayman, Pullman, WA CPC .................................. C07K5/06078 (2013.01) (US) USPC ........................................................... S14/95 (73) Assignee: Washington State University, Pullman, WA (US) (57) ABSTRACT (21) Appl. No.: 14/038,973 (22) Filed: Sep. 27, 2013 Small molecule, peptidic hepatocyte growth factors mimics, which act as both mimetics and antagonists, have been gen Related U.S. Application Data erated. These molecules have been shown or predicted to have (63) Continuation-in-part of application No. PCT/US2012/ therapeutic potential for numerous pathologies including 031815, filed on Apr. 2, 2012. dementia (e.g. Alzheimer's) and Parkinson's disease. Patent Application Publication Feb. 20, 2014 Sheet 1 of 40 US 2014/0051633 A1 a --- --- - - -s ------- CS -8- Scopolarine s -k- Oii exa-ow " -: N. Sks s i. Y s re. Dihexa-high as: ^ N.-- st-scs:- -------------------------------------------------------------------------------------------. Acquisition (days) Figure 1A -8-CSF -- -8- Scopoanine i. --- - -k- Dihexa-ow -- hexa-high reta-or 2 3 4 5 6 7 8 Acquisition (days) Figure 1B -8- vici-treated -- hexa Acquisition (days) Figure 1C Patent Application Publication Feb. -

Section 9. Physical and Chemical Properties
POLYONE CORPORATION SAFETY DATA SHEET AMPVC 325784 Version Number 1.0 Page 1 of 15 Revision Date 05/22/2020 Print Date 05/23/2020 SAFETY DATA SHEET AMPVC 325784 Section 1. Identification GHS product identifier : AMPVC 325784 Chemical name : Mixture CAS number : Mixture Other means of identification : CC10325784 Product type : solid Relevant identified uses of the substance or mixture and uses advised against Product use : Industrial applications. Plastics. Supplier's details : POLYONE CORPORATION 33587 Walker Road, Avon Lake, OH 44012 1 (440) 930-1000 or 1 (866) POLYONE Emergency telephone number : CHEMTREC 1-800-424-9300 (24hrs for spill, leak, fire, exposure or (with hours of operation) accident). Section 2. Hazards identification This mixture has not been evaluated as a whole for health effects. All ingredients are bound in a PVC polymer matrix and potential for hazardous exposure as shipped is minimal. PVC resin is manufactured from Vinyl Chloride Monomer (VCM). PVC resin manufacturers take special efforts to strip residual VCM from their resins. Residual VCM in the resin is typically below 8.5 ppm. However, VCM is a known carcinogen. The end-user (fabricator) should take necessary precautions (mechanical ventilation, local exhaust, respiratory protection, etc.) to protect employees from exposure to any vapors or dusts that may be released during heating or fabrication. See Sections 8 and 11 for special precautions.After handling, always wash hands thoroughly with soap and water. OSHA/HCS status : While this material is not considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200), this SDS contains valuable information critical to the safe handling and proper use of the product. -
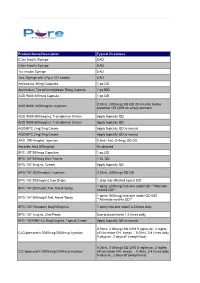
Pure Pricing List
Product Name/Description Typical Directions 0.3cc Insulin Syringe UAD 0.5cc Insulin Syringe UAD 1cc Insulin Syringe UAD 1mL Syringe with 27g x 1/2" needle UAD Amlexanox 40mg Capsules 1 po QD Ammonium Tetrathiomolybdate 50mg Capsule 1 po BID AOD 9604 600mcg Capsule 1 po QD 0.25mL (300mcg) SQ QD 30 minutes before AOD 9604 1200mcg/mL Injection breakfast OR QHS on empty stomach AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD/BPC 2mg/2mg Cream Apply topically QD to wound AOD/BPC 2mg/2mg Cream Apply topically QD to wound ARA 290 4mg/mL Injection 0.5mL-1mL (2-4mg) SQ QD Ascorbic Acid 500mg/mL As directed BPC-157 500mcg Capsules 1 po QD BPC-157 500mcg Mini-Troche 1 SL QD BPC-157 2mg/mL Cream Apply topically QD BPC-157 2000mcg/mL Injection 0.25mL (500mcg) SQ QD BPC-157 200mcg/mL Eye Drops 1 drop into affected eye(s) BID 1 spray (200mcg) into one nostril QD **Alternate BPC-157 200mcg/0.1mL Nasal Spray nostrils QD** 1 spray (500mcg) into one nostril QD-BID BPC-157 500mcg/0.1mL Nasal Spray **Alternate nostrils QD** BPC-157/ Synapsin 5mg/50mg/mL 1 spray into one nostril 2-3 times daily BPC-157 1mg/mL Oral Paste Use to brush teeth 1-2 times daily BPC-157/GHK-Cu 2mg/2mg/mL Topical Cream Apply topically QD to wound 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, sleep)… 0.05mL 3-4 times daily 5 days on, 2 days off (weight loss) 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, -

Counteracting EMF Toxicity with Peptides (Forget Global Warming Emfs Are Killing Us)
Counteracting EMF Toxicity with Peptides (Forget Global Warming EMFs are Killing Us) Dr. Kent Holtorf, MD Medical Director/CEO, Holtorf Medical Group (HoltorfMed.com) Medical Director/CEO, National Academy of Hypothyroidism (NAHypothyroidism.org) Medical Director, Integrative Peptides (IntegrativePeptides.com) Disclosure Statement Owner/CEO - Holtorf Medical Group HoltorfMed.com A multi-specialty medical group specializing in the treatment of complex endocrine dysfunction, CFS/fibromyalgia, chronic infectious diseases, immune dysfunction, neurodegenerative disease, and other complex chronic illnesses. Chief Medical Officer - The Non-Profit, The National Academy of Hypothyroidism and Integrative Sciences (NAHIS) NAHypothyroidism.org NAHIS is a non- profit, multidisciplinary medical society dedicated to the dissemination of new information on the diagnosis and treatment of hypothyroidism and complex conditions. NAHIS receives grants for clinical and laboratory research for novel methods in the diagnosis and treatment of hypothyroidism and chronic illnesses Chief Medical Officer - Integrative Peptides IntegrativePeptides.com Currently sells orally active and absorbable natural peptides in oral supplement form with unique delivery methods 2 Disclosure Statement Important Disclaimer In the interest of full disclosure, I am the All studies have limitations, and there is no perfect study. As with other studies, the studies Chief Medical Officer of Integrative Peptides. mentioned in this presentation and during the summit have limitations that should be considered when evaluating the efficacy of any treatment, including peptides, and determining the The opinions expressed are mine and do not appropriateness of peptide therapy for consumer use. A short summary, the abstract or reference to necessarily reflect those of Integrative a study may be discussed or provided. We are happy to provide you the entire study for your review of any study discussed, mentioned, presented or referenced. -

PEPTIDE INFORMATION Contact Kitt Burkley for Pricing 855-762-4878
PEPTIDE INFORMATION Contact Kitt Burkley for pricing 855-762-4878 AOD 9604 AOD 9604 works by mimicking the way natural HGH regulates fat metabolism but without the adverse effects on blood sugar or growth that is seen with unmodified HGH. Studies have suggested that AOD 9604 has an ability to stimulate lipolysis and inhibit lipogenesis. In addition AOD 9604 possesses other regenerative properties associated with growth hormone. Trials have been undertaken to determine the possible application of AOD 9604 in osteoarthritis and hypercholesterolemia, as well as for bone and cartilage repair. Results of oral glucose tolerance testing have demonstrated that AOD 9604 has no negative effect on carbohydrate metabolism, and it does not affect serum IGF-1 levels. AOD 9604 has an excellent safety profile and is generally well tolerated. It has attained Human GRAS status in the US. AOD 9604 600mcg/ml Cream – 30g AOD 9604* 0.5mg Troche BPC-157 (Body Protection Compound) BPC-157 promotes muscle and tendon healing, increases angiogenesis and decreases inflammatory response. Produces more type 1 collagen and has excellent potential for diabetic wound healing. BPC- 157 is highly stable, resistant to both hydrolysis and enzyme digestion. It can be given orally as well as administered subcutaneously or intramuscularly – no carrier molecule is required. This peptide has significant anti-inflammatory modulating effects. BPC-157 promotes tissue healing through signaling pathways – it provides specific response to particular injury thru cell signaling. BPC- 157 can be used continuously as body protective, or for specific period to treat body injury. BCP-157 is in clinical trials for IBD. -

Section 2. Hazards Identification
POLYONE CORPORATION SAFETY DATA SHEET STAN-TONE HCC- AMPVC 184897 Version Number 1.3 Page 1 of 16 Revision Date 08/10/2016 Print Date 08/11/2016 SAFETY DATA SHEET STAN-TONE HCC- AMPVC 184897 Section 1. Identification GHS product identifier : STAN-TONE HCC- AMPVC 184897 Chemical name : Mixture CAS number : Mixture Other means of identification : FO20031738 Product type : liquid Relevant identified uses of the substance or mixture and uses advised against Product use : Industrial applications. Plastics. Supplier's details : POLYONE CORPORATION 1675 Navarre Road SW, Massillon, Ohio USA 44646 1 330 837 8679 Emergency telephone number : CHEMTREC 1-800-424-9300 (24hrs for spill, leak, fire, exposure or (with hours of operation) accident). Section 2. Hazards identification This mixture has not been evaluated as a whole. Information provided on the health effects of this product is based on individual components. Some vapors may be released upon heating and the end-user (fabricator) must take the necessary precautions (mechanical ventilation, respiratory protection, etc.) to protect employees from exposure.After handling, always wash hands thoroughly with soap and water. OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the substance or : ACUTE TOXICITY (oral) - Category 4 mixture ACUTE TOXICITY (dermal) - Category 4 ACUTE TOXICITY (inhalation) - Category 4 SERIOUS EYE DAMAGE/ EYE IRRITATION - Category 2B GHS label elements 1/16 POLYONE CORPORATION SAFETY DATA SHEET STAN-TONE HCC- AMPVC 184897 Version Number 1.3 Page 2 of 16 Revision Date 08/10/2016 Print Date 08/11/2016 Hazard pictograms : Signal word : Warning Hazard statements : Harmful if swallowed, in contact with skin or if inhaled.