WO 2009/060024 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Repertorium Plantarum Succulentarum LIV (2003) Repertorium Plantarum Succulentarum LIV (2003)
ISSN 0486-4271 IOS Repertorium Plantarum Succulentarum LIV (2003) Repertorium Plantarum Succulentarum LIV (2003) Index nominum novarum plantarum succulentarum anno MMIII editorum nec non bibliographia taxonomica ab U. Eggli et D. C. Zappi compositus. International Organization for Succulent Plant Study Internationale Organisation für Sukkulentenforschung December 2004 ISSN 0486-4271 Conventions used in Repertorium Plantarum Succulentarum — Repertorium Plantarum Succulentarum attempts to list, under separate headings, newly published names of succulent plants and relevant literature on the systematics of these plants, on an annual basis. New names noted after the issue for the relevant year has gone to press are included in later issues. Specialist periodical literature is scanned in full (as available at the libraries at ZSS and Z or received by the compilers). Also included is information supplied to the compilers direct. It is urgently requested that any reprints of papers not published in readily available botanical literature be sent to the compilers. — Validly published names are given in bold face type, accompanied by an indication of the nomenclatu- ral type (name or specimen dependent on rank), followed by the herbarium acronyms of the herbaria where the holotype and possible isotypes are said to be deposited (first acronym for holotype), accord- ing to Index Herbariorum, ed. 8 and supplements as published in Taxon. Invalid, illegitimate, or incor- rect names are given in italic type face. In either case a full bibliographic reference is given. For new combinations, the basionym is also listed. For invalid, illegitimate or incorrect names, the articles of the ICBN which have been contravened are indicated in brackets (note that the numbering of some regularly cited articles has changed in the Tokyo (1994) edition of ICBN). -
2012 Formatted Lists
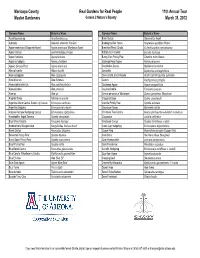
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

September 2013
SCCSS South Coast Cactus & Succulent Society Prickly News South Coast Cactus & Succulent Society Newsletter - September 2013 General MeetinG president’s Message Sunday - September 8, 1:30 pm august 2013 We will meet in the Hall Setting up for last month’s “Baja California and opuntioid diverSity”– meeting started badly. First there Presentation by was no projector for our speaker Jon P. Rebman, Ph.D., and then the new wireless mi- Curator of Botany, crophones worked fine, but the San Diego Natural amplifier didn’t, and neither did History Museum the backup system. But, while I put business before pleasure, Laurel Woodley graciously The desert regions of Baja returned home to pick up her projector for our speaker’s California and southern program. So, all’s well that ends well, as they say California satisfy my need for scientific adventure and our speaker, Gunner Eisel, showed us what can while providing a sense of excitement towards be done in the way of modifying an Astrophytum. We botany, reverence for nature and its unaltered beauty, saw some truly amazing plants. appreciation for the complexity of natural history, In upcoming issues, you may (or may not) see and an overall feeling of peace and purpose. articles submitted by members to our editor, Judi Woo- — Jon P. Rebman Sato, for publication. If you have something relevant to the club or hobby that you’d like to share, you may inSide thiS iSSue submit your article for publication consideration. President’s Message 1 Depending on available space, Judi will decide Refreshments 1 whether and when to publish. -

Cactoblastis Cactorum) Detection and Monitoring Network
Survey Information for the National Cactus Moth (Cactoblastis cactorum) Detection and Monitoring Network Joel Floyd USDA, APHIS, PPQ, Riverdale, MD John D. Madsen GeoResources Institute, Mississippi State University, Mississippi State, MS GeoResources Institute, Mississippi State, MS 39762-9652 GeoResources Institute Report #5013 April 23, 2007 Mississippi State University Page 1 April 23, 2007 Sponsors and Cooperators FEDERAL US Department of Agriculture APHIS PPQ US Department of Agriculture Agricultural Research Service US Geological Survey, Biological Resources Discipline National Biological Information Infrastructure US Forest Service US National Park Service US Army Corps of Engineers US Fish and Wildlife Service US Bureau of Land Management STATE Mississippi Department of Agriculture and Commerce, Bureau of Plant Industry UNIVERSITIES Mississippi State University Mississippi State University-Extension Service OTHER GROUPS Master Gardeners of Mississippi Cover illustration by Joel Floyd Mississippi State University Page 2 April 23, 2007 Survey Information for the National Cactus Moth (Cactoblastis cactorum) Detection and Monitoring Network Joel Floyd, USDA, APHIS, PPQ, Riverdale, MD John Madsen,GeoResources Institute, Mississippi State University, Mississippi State, MS Background: Cactoblastis cactorum Berg. was introduced into Australia from Argentina in the 1920’s to control exotic invasive prickly pear cacti. The program was phenomenally successful at reducing large stands of prickly pear in a relatively short period of time. Over the next several decades, C. cactorum was then taken to other parts of the world to control invasive prickly pear, including Hawaii in 1950 and the Caribbean island of Nevis in 1956. Spreading from Nevis to other islands, C. cactorum was eventually detected in the Florida Keys in 1989. -

Cactus Island
CACTUS ISLAND AFRICA - AMERICA - MADAGASCAR AMERICA’S CACTUS ISLAND This Agave is a spectacular hybrid of green leaves with a red margin. It can be planted in full sun or in sun-shade. It should be planted in well-drained soil and watered once a month. During winter it is advisable not to water it too much to increase its resistance to cold. It is advisable to avoid that the snow accumulates between the leaves. Tª min approx -8 / -10 ºC. AGAVE ARISTOCRAT Agave salmiana var. ferox (Agave ferox) is a variety of the agave of the Salmiana species belonging to the genus Agave and family Asparagaceae. The ferox subspecies is very close to the typical subspecies in terms of description and culture. It differs by having even thicker and stiffer blades, pointed ends (up to 8 cm) and even sharper lateral spines. This is why it is called by that name. Place of origin: Mexico. AGAVE FEROX NOTES: Agaves tolerate arid and semi-desert areas well, in fact, they are usually the only survivors in abandoned gardens. Ideal for plantations that do not need care or irrigation. They prefer sunny and airy places, with well-drained soils. Very little or no watering if the plant grows in full soil. In a pot, they should be watered but very little. Agave lophantha is a plant native to the deserts of Chihuahua and Sonora. It forms a rosette of coriaceous leaves or leaves up to 45cm high and 60cm wide, with jagged edges. The flowers appear grouped in reddish-yellow inflorescences up to 4m high. -

Biodiversity and Conservation Volume 8 Number 12, December 2016 ISSN 2141-243X
International Journal of Biodiversity and Conservation Volume 8 Number 12, December 2016 ISSN 2141-243X ABOUT IJBC The International Journal of Biodiversity and Conservation (IJBC) (ISSN2141-243X) is published Monthly (one volume per year) by Academic Journals. International Journal of Biodiversity and Conservation (IJBC)provides rapid publication (monthly) of articles in all areas of the subject such as Information Technology and its Applications in Environmental Management and Planning, Environmental Management and Technologies, Green Technology and Environmental Conservation, Health: Environment and Sustainable Development etc. The Journal welcomes the submission of manuscripts that meet the general criteria of significance and scientific excellence. Papers will be published shortly after acceptance. All articles published in IJBC are peer reviewed. Contact Us Editorial Office: [email protected] Help Desk: [email protected] Website: http://www.academicjournals.org/journal/IJBC Submit manuscript online http://ms.academicjournals.me/ Editor-In-Chief Associate Editors Prof. Samir I. Ghabbour Dr. Shannon Barber-Meyer Department of Natural Resources, World Wildlife Fund Institute of African Research & Studies, Cairo 1250 24th St. NW, Washington, DC 20037 University, Egypt USA Dr. Shyam Singh Yadav Editors National Agricultural Research Institute, Papua New Guinea Dr. Edilegnaw Wale, PhD Department of Agricultural Economics Schoolof Agricultural Sciences and Agribusiness Dr. Michael G. Andreu University of Kwazulu-Natal School of Forest Resources and Conservation P bag X 01 Scoffsville 3209 University of Florida - GCREC Pietermaritzburg 1200 N. Park Road South Africa. Plant City, FL USA Dr. BeqirajSajmir Department of Biology Dr. S.S. Samant Faculty of Natural Sciences, Biodiversity Conservation and Management University of Tirana G>B. Pant Institute of Himalayan BulevardiZog I, Tirana, Environment and Development, Albania Himachal Unit, Mohal-Kullu- 175 126, Himachal Pradesh, India Dr. -

Dry Country Plants of the South Texas Plains
Dry Country Plants of the South Texas Plains Item Type Article Authors Crosswhite, Frank S. Publisher University of Arizona (Tucson, AZ) Journal Desert Plants Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 23/09/2021 18:50:23 Link to Item http://hdl.handle.net/10150/550764 Crosswhite South Texas Plains 141 The semi -desert South Texas Plains vegetational Dry Country Plants division today encompasses large acreages of fruits and vegetables along the lower Rio Grande where irrigation is of the South Texas Plains possible and vast cattle ranches in the many square miles where water is more scarce. The present article discusses 1) the history of the region in relation to the native plants, 2) the uses of the native plants for food, fiber, shelter and Frank S. Crosswhite medicine, 3) the valuable native grasses which provided Boyce Thompson Southwestern Arboretum forage for beginning the cattle industry in America, 4) the and Department of Plant Sciences, Tamaulipan Brushland element which provided habitat University of Arizona for mother cow giving birth to call, 5) the vegetation of the resacas and floodplain of the lower Rio Grande before con- version to fruits and vegetables, and 6) the native plants which have potential uses in landscaping. The Spanish explorer Cabeza de Vaca passed through the region in 1535. The Indians gave him clothing made of Algodón (Cotton, Gossypium hirsutum), a fiber which was to become a leading crop in Texas during the next 400 years. During World War II, the federal government or- dered ten million acres of cotton land converted to the raising of feed for livestock, and after the war much of the land was never returned to cotton but made to yield human food. -

Edible Landscape, Q & A
The Blooming Bell July, 2012 BCMGA Newsletter Inside this edition: July Calendar, President’s Corner, Dallas Arboretum Remembering Laverne Edible Landscape, Q & A Cactus Care Educational Opportunities Announcements, What’s Happening in Your Yard July 2012 Sun Mon Tue Wed Thu Fri Sat 1 2 3 4 5 6 7 NO Burger Wednesday 8 9 10 11 12 13 14 Work Day 7 - 8:30 am General Meeting, Clint Walker speaks about Bees and his recent trip to Africa 11 am 15 16 17 18 19 20 21 22 23 24 25 26 27 28 Work Day Keyhole 7 - 8:30am Gardening, 9-11am at the Board of Directors Blackland Meeting 9 am Research Center 29 30 31 Upcoming Dates to Remember: Fall Veggie and Tree Planting Seminar, September 15th Fall Plant Sale is September 22nd Fall Garden Tour, October 20 Flag image by Nuttakit of Freedigitalimages.net 2 The “Welcome Home Garden” project is in need of The President’s Corner one or two more veterans to volunteer. The program is being coordinated locally by Texas Military 4-H at Summer has definitely arrived. I took an early morning Blackland. There are several different directions the walk to my vegetable garden, watered the pot plants, volunteers can go with the project. Please contact me filled the bird baths, picked the figs and returned to if you would be interested in helping. The project can the comfort of the indoors. Now I will get down to do- be simple or grand in scale; it will be up to the volun- ing all those indoor projects I have been putting off teers. -

Juli 1996 Jahrgang 47 ISSN 0022 7846
Heft 7 - Juli 1996 - 47. Jahrgang H6000 Kakteen und andere und Sukkulenten Kakteen Kakteen und andere Sukkulenten INHALT monatlich erscheinendes Organ der als Herausgeber genannten Gesellschaften Heft 7 Juli 1996 Jahrgang 47 ISSN 0022 7846 Artenschutz DETLEV METZING WERNER HOFFMANN Editorial Kakteen für Bolivien 141 Echinopsis formosa (Pfeiffer) Salm-Dyck [syn. Trichocereus tarijensis (Vaupel) Werdermann] aus Bolivien ziert blühend die Titelsei Im Habitat te dieses Hefts. Der dazugehörige Beitrag zeigt einen wegweisenden NORBERT F. A. ZIMMERMANN Frithia pulchra N. E. Brown - Ansatz in den Bemühungen um den Schutz der Kakteen und ihrer Eine Reise zu zwei Populationen im Lebensräume. Nicht in unseren Sammlungen müssen Kakteen langfri Transvaal mit Besprechung der stig überleben, sondern in ihren heimatlichen Lebensräumen. Dies sukkulenten Begleitvegetation 146 kann aber nur gelingen, wenn in den Herkunftsländern der Kakteen Pflegetips das Wissen über die eigene Flora gefördert wird. Manche Länder haben DIETER HERBEL nur beschränkte Mittel zur Verfügung. Gerade Bolivien beherbergt nach Biologischer Pflanzenschutz bei Mexiko die reichste Kakteenflora überhaupt. Das Wissen darüber wird Kakteen und anderen Sukkulenten - erfolgreicher Einsatz von Nützlingen 153 jedoch in Europa und den USA gehütet und ist für Bolivianer de facto unzugänglich. Das hier vorgestellte Projekt bemüht sich, diese gravie Nomenklatur renden Schranken abzubauen. Zur Finanzierung trug übrigens in WALTER WESKAMP nachahmenswerter Weise auch die Ortsgruppe „Rheingau“ der DKG Parodia winbergii Weskamp spec. nov. 157 bei. Wir stellen vor Aus dem weiteren Inhalt: Die „anderen Sukkulenten“ sind diesmal JONAS LÜTHY durch einen Beitrag über die Gattung Frithia aus der Familie der Opuntia basilaris und Aizoaceen oder Mittagsblumengewächse vertreten. Beobachtungen an Yucca brevifolia 160 zwei bekannten Populationen von F. -

GREATER PHOENIX REGIONAL ATLAS a Preview of the Region’S 50-Year Future GP2100 ACKNOWLEDGMENTS
GREATER PHOENIX REGIONAL ATLAS A Preview of the Region’s 50-Year Future GP2100 ACKNOWLEDGMENTS GREATER PHOENIX 2100 STEERING COMMITTEE Ray Quay, Atlas Editor Jonathan Fink Grady Gammage, Jr. Rob Melnick Charles Redman Fritz Steiner, Emeritus COMMENTATORS REVIEWERS Catherine R. Eden, Arizona Department of Health Services Eric Anderson, Maricopa Association of Governments Editorial Board, The Arizona Republic Lindy Bauer, Maricopa Association of Governments Ed Fox, Pinnacle West Diane Bender, Arizona State University Terry Goddard, Arizona Attorney General, Formerly U.S. Department of Housing and Urban Development, Arizona State Office Lynn Favour, Maricopa County Planning & Development Grady Gammage, Jr., Central Arizona Project Will Humble, Arizona Department of Health Services Sheila Grinell, Arizona Science Center Mary Kihl, Arizona State University Ed Pastor, Fourth Congressional District of Arizona Dale Mason, Arizona Department of Water Resources Dr. Carol Peck, The Rodel Charitable Foundation of Arizona, Jim Mathien, City of Phoenix Formerly Alhambra Elementary School District Fernando Munoz-Carmona, Arizona State University Charles L. Redman, Arizona State University Jon Talton, The Arizona Republic Steve Rossi, City of Phoenix Rick Weddle, Greater Phoenix Economic Council Rita Walton, Maricopa Association of Governments Raymond L. Woosley, The University of Arizona Harry Wolfe, Maricopa Association of Governments ISSUE AND DATA DEVELOPMENT Anubhav Bagley, Maricopa Association of Governments Dale Mason, Arizona Department of Water Resources Anthony Brazel, Arizona State University Peter McCartney, Arizona State University Jay Butler, Arizona State University Jennifer McCulley, Arizona State University Ruey-In Chiou, Maricopa Association of Governments Christine McRight, Nathan & Associates, Inc. Phil Cummings, Maricopa County Laura Musacchio, Arizona State University Marta Dent, Flood Control District of Maricopa County Tom Rex, Arizona State University H. -

Stimulation of Desert Dry Country Plants of the Arboretum Progress
Desert Plants, Volume 2, Number 3 (Autumn 1980) Item Type Article Publisher University of Arizona (Tucson, AZ) Journal Desert Plants Rights Copyright © Arizona Board of Regents. The University of Arizona. Download date 09/10/2021 02:10:07 Link to Item http://hdl.handle.net/10150/550746 Volume 2. Number 3. Autumn 1980 Desert Published by The University of Arizona for the Plants Boyce Thompson Southwestern Arboretum Editorial - Stimulation of Desert Plant Research in the United States as a Little -known Result of the Russian Revolution of 1917 140 Dry Country Plants of the South Texas Plains Frank S. Crosswhite 141 Arboretum Progress 180 Distribution of the Boojum Tree, Idria columnaris, on the Coast of Sonora, Mexico as Influenced by Climate 183 Robert R. Humphrey and David B. Marx Ammobroma sonorae, an Endangered Parasitic Plant in Extremely Arid North America 188 Gary Nabhan Biomass Potential in Arizona 197 Kennith E. Foster, R. Leslie Rawles and Martin M. Karpiscak Reviews 201 Booium Trees (Idria columnaris) Drawing by Roberta January Humphrey see article on page 183 Subscriptions to Desert Plants To obtain a one -year subscription to Desert Plants, just send Sl0 to the address below Desert Plants Boyce Thompson SW Arboretum P.O. Box AB Superior, Arizona 85273 Make sure you provide your current address with zip code. If the subscription isa renewal, please say so to avoid receiving two copies of the same issue. In the case of gift subscriptions, we will send a letter to the recipient telling who is sending the gift, if this is requested. Of course, no extra charge. -

Repertorium Plantarum Succulentarum LXIII (2012) Ashort History of Repertorium Plantarum Succulentarum
ISSN 0486-4271 Inter national Organization forSucculent Plant Study Organización Internacional paraelEstudio de Plantas Suculentas Organisation Internationale de Recherche sur les Plantes Succulentes Inter nationale Organisation für Sukkulenten-Forschung Repertorium Plantarum Succulentarum LXIII (2012) Ashort history of Repertorium Plantarum Succulentarum The first issue of Repertorium Plantarum Succulentarum (RPS) was produced in 1951 by Michael Roan (1909 −2003), one of the founder members of the International Organization for Succulent Plant Study (IOS) in 1950. It listed the ‘majority of the newnames [of succulent plants] published the previous year’. The first issue, edited by Roan himself with the help of A. J. A Uitewaal (1899 −1963), was published for IOS by the National Cactus & Succulent Society,and the next four (with Gordon RowleyasAssociate and later Joint Editor) by Roan’snewly formed British Section of the IOS. For issues 5 − 12, Gordon Rowleybecame the sole editor.Issue 6 was published by IOS with assistance by the Acclimatisation Garden Pinya de Rosa, Costa Brava,Spain, owned by Fernando Riviere de Caralt (1904 −1992), another founder member of IOS. In 1957, an arrangement for closer cooperation with the International Association of Plant Taxonomy (IAPT) was reached, and RPS issues 7−22 were published in their Regnum Ve getabile series with the financial support of the International Union of Biological Sciences (IUBS), of which IOS remains a member to this day.Issues 23−25 were published by AbbeyGarden Press of Pasadena, California, USA, after which IOS finally resumed full responsibility as publisher with issue 26 (for 1975). Gordon Rowleyretired as editor after the publication of issue 32 (for 1981) along with Len E.