New World Cactaceae Plants Harbor Diverse Geminiviruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Repertorium Plantarum Succulentarum LIV (2003) Repertorium Plantarum Succulentarum LIV (2003)
ISSN 0486-4271 IOS Repertorium Plantarum Succulentarum LIV (2003) Repertorium Plantarum Succulentarum LIV (2003) Index nominum novarum plantarum succulentarum anno MMIII editorum nec non bibliographia taxonomica ab U. Eggli et D. C. Zappi compositus. International Organization for Succulent Plant Study Internationale Organisation für Sukkulentenforschung December 2004 ISSN 0486-4271 Conventions used in Repertorium Plantarum Succulentarum — Repertorium Plantarum Succulentarum attempts to list, under separate headings, newly published names of succulent plants and relevant literature on the systematics of these plants, on an annual basis. New names noted after the issue for the relevant year has gone to press are included in later issues. Specialist periodical literature is scanned in full (as available at the libraries at ZSS and Z or received by the compilers). Also included is information supplied to the compilers direct. It is urgently requested that any reprints of papers not published in readily available botanical literature be sent to the compilers. — Validly published names are given in bold face type, accompanied by an indication of the nomenclatu- ral type (name or specimen dependent on rank), followed by the herbarium acronyms of the herbaria where the holotype and possible isotypes are said to be deposited (first acronym for holotype), accord- ing to Index Herbariorum, ed. 8 and supplements as published in Taxon. Invalid, illegitimate, or incor- rect names are given in italic type face. In either case a full bibliographic reference is given. For new combinations, the basionym is also listed. For invalid, illegitimate or incorrect names, the articles of the ICBN which have been contravened are indicated in brackets (note that the numbering of some regularly cited articles has changed in the Tokyo (1994) edition of ICBN). -

Beet Curly Top Virus Strains Associated with Sugar Beet in Idaho, Oregon, and a Western U.S
Plant Disease • 2017 • 101:1373-1382 • http://dx.doi.org/10.1094/PDIS-03-17-0381-RE Beet curly top virus Strains Associated with Sugar Beet in Idaho, Oregon, and a Western U.S. Collection Carl A. Strausbaugh and Imad A. Eujayl, United States Department of Agriculture–Agricultural Research Service (USDA-ARS) Northwest Irrigation and Soils Research Laboratory, Kimberly, ID 83341; and William M. Wintermantel, USDA-ARS, Salinas, CA 93905 Abstract Curly top of sugar beet is a serious, yield-limiting disease in semiarid pro- Logan) strains and primers that amplified a group of Worland (Wor)- duction areas caused by Beet curly top virus (BCTV) and transmitted like strains. The BCTV strain distribution averaged 2% Svr, 30% CA/ by the beet leafhopper. One of the primary means of control for BCTV Logan, and 87% Wor-like (16% had mixed infections), which differed in sugar beet is host resistance but effectiveness of resistance can vary from the previously published 2006-to-2007 collection (87% Svr, 7% among BCTV strains. Strain prevalence among BCTV populations CA/Logan, and 60% Wor-like; 59% mixed infections) based on a contin- was last investigated in Idaho and Oregon during a 2006-to-2007 collec- gency test (P < 0.0001). Whole-genome sequencing (GenBank acces- tion but changes in disease severity suggested a need for reevaluation. sions KT276895 to KT276920 and KX867015 to KX867057) with Therefore, 406 leaf samples symptomatic for curly top were collected overlapping primers found that the Wor-like strains included Wor, Colo- from sugar beet plants in commercial sugar beet fields in Idaho and rado and a previously undescribed strain designated Kimberly1. -

Geminiviruses Encode Additional Small Proteins with Specific
ARTICLE https://doi.org/10.1038/s41467-021-24617-4 OPEN Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function Pan Gong 1,6, Huang Tan 2,3,6, Siwen Zhao1, Hao Li1, Hui Liu4,YuMa2,3, Xi Zhang2,3, Junjie Rong2,3, Xing Fu2, ✉ ✉ ✉ Rosa Lozano-Durán 2,5 , Fangfang Li1 & Xueping Zhou 1,4 1234567890():,; Geminiviruses are plant viruses with limited coding capacity. Geminivirus-encoded proteins are traditionally identified by applying a 10-kDa arbitrary threshold; however, it is increasingly clear that small proteins play relevant roles in biological systems, which calls for the reconsideration of this criterion. Here, we show that geminiviral genomes contain additional ORFs. Using tomato yellow leaf curl virus, we demonstrate that some of these small ORFs are expressed during the infection, and that the encoded proteins display specific subcellular localizations. We prove that the largest of these additional ORFs, which we name V3, is required for full viral infection, and that the V3 protein localizes in the Golgi apparatus and functions as an RNA silencing suppressor. These results imply that the repertoire of gemi- niviral proteins can be expanded, and that getting a comprehensive overview of the molecular plant-geminivirus interactions will require the detailed study of small ORFs so far neglected. 1 State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China. 2 Shanghai Center for Plant Stress Biology, CAS Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai, China. 3 University of the Chinese Academy of Sciences, Beijing, China. -
2012 Formatted Lists
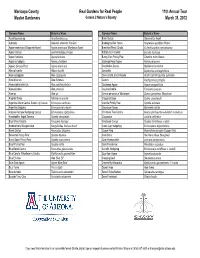
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Diversity and Evolution of Viral Pathogen Community in Cave Nectar Bats (Eonycteris Spelaea)
viruses Article Diversity and Evolution of Viral Pathogen Community in Cave Nectar Bats (Eonycteris spelaea) Ian H Mendenhall 1,* , Dolyce Low Hong Wen 1,2, Jayanthi Jayakumar 1, Vithiagaran Gunalan 3, Linfa Wang 1 , Sebastian Mauer-Stroh 3,4 , Yvonne C.F. Su 1 and Gavin J.D. Smith 1,5,6 1 Programme in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore 169857, Singapore; [email protected] (D.L.H.W.); [email protected] (J.J.); [email protected] (L.W.); [email protected] (Y.C.F.S.) [email protected] (G.J.D.S.) 2 NUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, Singapore 119077, Singapore 3 Bioinformatics Institute, Agency for Science, Technology and Research, Singapore 138671, Singapore; [email protected] (V.G.); [email protected] (S.M.-S.) 4 Department of Biological Sciences, National University of Singapore, Singapore 117558, Singapore 5 SingHealth Duke-NUS Global Health Institute, SingHealth Duke-NUS Academic Medical Centre, Singapore 168753, Singapore 6 Duke Global Health Institute, Duke University, Durham, NC 27710, USA * Correspondence: [email protected] Received: 30 January 2019; Accepted: 7 March 2019; Published: 12 March 2019 Abstract: Bats are unique mammals, exhibit distinctive life history traits and have unique immunological approaches to suppression of viral diseases upon infection. High-throughput next-generation sequencing has been used in characterizing the virome of different bat species. The cave nectar bat, Eonycteris spelaea, has a broad geographical range across Southeast Asia, India and southern China, however, little is known about their involvement in virus transmission. -

Ocorrência Natural De Espécies Virais E Avaliação De Potenciais Hospedeiras Experimentais De Begomovírus, Potyvírus E Tospovírus Em Plantas Arbóreas E Berinjela
UNIVERSIDADE DE BRASÍLIA INSTITUTO DE CIÊNCIAS BIOLÓGICAS DEPARTAMENTO DE FITOPATOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM FITOPATOLOGIA Ocorrência natural de espécies virais e avaliação de potenciais hospedeiras experimentais de begomovírus, potyvírus e tospovírus em plantas arbóreas e berinjela (Solanum melongena) JOSIANE GOULART BATISTA Brasília - DF 2014 JOSIANE GOULART BATISTA Ocorrência natural de espécies virais e avaliação de potenciais hospedeiras experimentais de begomovírus, potyvírus e tospovírus em plantas arbóreas e berinjela (Solanum melongena) Dissertação apresentada à Universidade de Brasília como requisito parcial para a obtenção do título de Mestre em Fitopatologia pelo Programa de Pós Graduação em Fitopatologia. Orientadora Dra. Rita de Cássia Pereira Carvalho BRASÍLIA DISTRITO FEDERAL – BRASIL 2014 FICHA CATALOGRÁFICA Batista, G.J. Ocorrência natural de espécies virais e avaliação de potenciais hospedeiras experimentais de begomovírus, potyvírus e tospovírus em plantas arbóreas e berinjela (Solanum melongena). Josiane Goulart Batista. Brasília, 2014 Número de páginas p.: 183 Dissertação de mestrado - Programa de Pós-Graduação em Fitopatologia, Universidade de Brasília, Brasília. Árvores, berinjela, vírus, resistência. I. Universidade de Brasília. PPG/FIT. II. Título. Ocorrência natural de espécies virais e avaliação de potenciais hospedeiras experimentais de begomovírus, potyvírus e tospovírus em plantas arbóreas e berinjela (Solanum melongena). Dedico a minha pequena linda filha Giovanna, ao meu pai Josvaldo, a minha mãe Nelma, ao meu namorado Airton, a minha irmã Josefa e minha sobrinha Júlia. Agradecimentos A Deus. Aos meus pais Josvaldo e Nelma. A minha filha Giovanna, minha irmã Josefa e minha sobrinha Júlia. A minha orientadora Professora Rita de Cássia. Aos colegas do mestrado e doutorado Rafaela, Marcella, Juliana, Ícaro, Cléia, Monica, Elenice, William, Rayane e Nédio. -

Biological and Molecular Characterization of Dahlia Mosaic Caulimovirus Abstract
BIOLOGICAL AND MOLECULAR CHARACTERIZATION OF DAHLIA MOSAIC CAULIMOVIRUS By VIHANGA PAHALAWATTA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Plant Pathology AUGUST 2007 © Copyright by VIHANGA PAHALAWATTA, 2007 All Rights Reserved i To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of VIHANGA PAHALAWATTA find it satisfactory and recommend that it be accepted. _____________________________ Chair _____________________________ _____________________________ ____________________________ ii ACKNOWLEDGEMENT I would like to express my sincere gratitude to my major advisor, Dr. Hanu Pappu, for the tremendous support, guidance, encouragement and most of all the numerous opportunities that he made available to me during the time I spent working with him. Dr. Pappu has been an exceptional mentor who has been a constant source of inspiration to me. I would also like to thank Dr. Patricia Okubara, Dr. Ken Eastwell and Dr. Gary Chastagner for their advice, guidance and helpful discussions throughout my tenure. I wish to extend my gratitude to Keri Druffel, who taught me numerous techniques in the laboratory and for all the work she did that made my work so much easier. A special thanks to Robert Brueggeman for technical assistance. I am also grateful to the faculty and staff of the Department of Plant Pathology for all the help and support during my graduate studies at Washington State University. A special thank you to Dr. Tim Murray, for arranging departmental financial support and for giving me the opportunity to serve as a teaching assistant. -

September 2013
SCCSS South Coast Cactus & Succulent Society Prickly News South Coast Cactus & Succulent Society Newsletter - September 2013 General MeetinG president’s Message Sunday - September 8, 1:30 pm august 2013 We will meet in the Hall Setting up for last month’s “Baja California and opuntioid diverSity”– meeting started badly. First there Presentation by was no projector for our speaker Jon P. Rebman, Ph.D., and then the new wireless mi- Curator of Botany, crophones worked fine, but the San Diego Natural amplifier didn’t, and neither did History Museum the backup system. But, while I put business before pleasure, Laurel Woodley graciously The desert regions of Baja returned home to pick up her projector for our speaker’s California and southern program. So, all’s well that ends well, as they say California satisfy my need for scientific adventure and our speaker, Gunner Eisel, showed us what can while providing a sense of excitement towards be done in the way of modifying an Astrophytum. We botany, reverence for nature and its unaltered beauty, saw some truly amazing plants. appreciation for the complexity of natural history, In upcoming issues, you may (or may not) see and an overall feeling of peace and purpose. articles submitted by members to our editor, Judi Woo- — Jon P. Rebman Sato, for publication. If you have something relevant to the club or hobby that you’d like to share, you may inSide thiS iSSue submit your article for publication consideration. President’s Message 1 Depending on available space, Judi will decide Refreshments 1 whether and when to publish. -

Virus World As an Evolutionary Network of Viruses and Capsidless Selfish Elements
Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Koonin, E. V., & Dolja, V. V. (2014). Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements. Microbiology and Molecular Biology Reviews, 78(2), 278-303. doi:10.1128/MMBR.00049-13 10.1128/MMBR.00049-13 American Society for Microbiology Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Eugene V. Koonin,a Valerian V. Doljab National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland, USAa; Department of Botany and Plant Pathology and Center for Genome Research and Biocomputing, Oregon State University, Corvallis, Oregon, USAb Downloaded from SUMMARY ..................................................................................................................................................278 INTRODUCTION ............................................................................................................................................278 PREVALENCE OF REPLICATION SYSTEM COMPONENTS COMPARED TO CAPSID PROTEINS AMONG VIRUS HALLMARK GENES.......................279 CLASSIFICATION OF VIRUSES BY REPLICATION-EXPRESSION STRATEGY: TYPICAL VIRUSES AND CAPSIDLESS FORMS ................................279 EVOLUTIONARY RELATIONSHIPS BETWEEN VIRUSES AND CAPSIDLESS VIRUS-LIKE GENETIC ELEMENTS ..............................................280 Capsidless Derivatives of Positive-Strand RNA Viruses....................................................................................................280 -

Sequences and Phylogenies of Plant Pararetroviruses, Viruses and Transposable Elements
Hansen and Heslop-Harrison. 2004. Adv.Bot.Res. 41: 165-193. Page 1 of 34. FROM: 231. Hansen CN, Heslop-Harrison JS. 2004 . Sequences and phylogenies of plant pararetroviruses, viruses and transposable elements. Advances in Botanical Research 41 : 165-193. Sequences and Phylogenies of 5 Plant Pararetroviruses, Viruses and Transposable Elements CELIA HANSEN AND JS HESLOP-HARRISON* DEPARTMENT OF BIOLOGY 10 UNIVERSITY OF LEICESTER LEICESTER LE1 7RH, UK *AUTHOR FOR CORRESPONDENCE E-MAIL: [email protected] 15 WEBSITE: WWW.MOLCYT.COM I. Introduction ............................................................................................................2 A. Plant genome organization................................................................................2 20 B. Retroelements in the genome ............................................................................3 C. Reverse transcriptase.........................................................................................4 D. Viruses ..............................................................................................................5 II. Retroelements........................................................................................................5 A. Viral retroelements – Retrovirales....................................................................6 25 B. Non-viral retroelements – Retrales ...................................................................7 III. Viral and non-viral elements................................................................................7 -

Cactoblastis Cactorum) Detection and Monitoring Network
Survey Information for the National Cactus Moth (Cactoblastis cactorum) Detection and Monitoring Network Joel Floyd USDA, APHIS, PPQ, Riverdale, MD John D. Madsen GeoResources Institute, Mississippi State University, Mississippi State, MS GeoResources Institute, Mississippi State, MS 39762-9652 GeoResources Institute Report #5013 April 23, 2007 Mississippi State University Page 1 April 23, 2007 Sponsors and Cooperators FEDERAL US Department of Agriculture APHIS PPQ US Department of Agriculture Agricultural Research Service US Geological Survey, Biological Resources Discipline National Biological Information Infrastructure US Forest Service US National Park Service US Army Corps of Engineers US Fish and Wildlife Service US Bureau of Land Management STATE Mississippi Department of Agriculture and Commerce, Bureau of Plant Industry UNIVERSITIES Mississippi State University Mississippi State University-Extension Service OTHER GROUPS Master Gardeners of Mississippi Cover illustration by Joel Floyd Mississippi State University Page 2 April 23, 2007 Survey Information for the National Cactus Moth (Cactoblastis cactorum) Detection and Monitoring Network Joel Floyd, USDA, APHIS, PPQ, Riverdale, MD John Madsen,GeoResources Institute, Mississippi State University, Mississippi State, MS Background: Cactoblastis cactorum Berg. was introduced into Australia from Argentina in the 1920’s to control exotic invasive prickly pear cacti. The program was phenomenally successful at reducing large stands of prickly pear in a relatively short period of time. Over the next several decades, C. cactorum was then taken to other parts of the world to control invasive prickly pear, including Hawaii in 1950 and the Caribbean island of Nevis in 1956. Spreading from Nevis to other islands, C. cactorum was eventually detected in the Florida Keys in 1989. -

ICTV Code Assigned: 2011.001Ag Officers)
This form should be used for all taxonomic proposals. Please complete all those modules that are applicable (and then delete the unwanted sections). For guidance, see the notes written in blue and the separate document “Help with completing a taxonomic proposal” Please try to keep related proposals within a single document; you can copy the modules to create more than one genus within a new family, for example. MODULE 1: TITLE, AUTHORS, etc (to be completed by ICTV Code assigned: 2011.001aG officers) Short title: Change existing virus species names to non-Latinized binomials (e.g. 6 new species in the genus Zetavirus) Modules attached 1 2 3 4 5 (modules 1 and 9 are required) 6 7 8 9 Author(s) with e-mail address(es) of the proposer: Van Regenmortel Marc, [email protected] Burke Donald, [email protected] Calisher Charles, [email protected] Dietzgen Ralf, [email protected] Fauquet Claude, [email protected] Ghabrial Said, [email protected] Jahrling Peter, [email protected] Johnson Karl, [email protected] Holbrook Michael, [email protected] Horzinek Marian, [email protected] Keil Guenther, [email protected] Kuhn Jens, [email protected] Mahy Brian, [email protected] Martelli Giovanni, [email protected] Pringle Craig, [email protected] Rybicki Ed, [email protected] Skern Tim, [email protected] Tesh Robert, [email protected] Wahl-Jensen Victoria, [email protected] Walker Peter, [email protected] Weaver Scott, [email protected] List the ICTV study group(s) that have seen this proposal: A list of study groups and contacts is provided at http://www.ictvonline.org/subcommittees.asp .