Short Course I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Bridge Between Science and the Future It Creates
Cell Stem Cell Resource Review Communicating Hope: the Bridge between Science and the Future It Creates The Stem Cell Hope: How Stem Cell Medicine Can Change Our Lives Alice Park New York, Hudson Street Press (2011) 336 pp., ISBN: 978-1-59463-078-1 Stem cells and hope. For those who place their futures in the perpetuation of biomedical research, these two constructs— stem cells and hope—have become nearly synonymous. For the scientific community, the potential for their work to be the basis of hope seems all too obvious. Science has long been the very vehicle by which society tackles its most pressing and perplexing challenges. It was Vannevar Bush, the first US presi- dential science advisor and the intellectual powerhouse behind the National Science Foundation, who championed the role that science would need to play in addressing broad social, the opponents’ court. Park notes that the Bush advisor Jay Lef- economic, and technological problems. However, it was also kowitz himself stated, ‘‘A lot of the conservative groups saw Vannevar Bush who stated, ‘‘A belief may be larger than this .a little bit as a surrogate for abortion.’’ Language that a fact,’’ and for stem cell research, this perceptive observation has framed the debate, such as ‘‘cloning,’’ ‘‘destruction,’’ made over 50 years ago has come to define the field. ‘‘fetus,’’ ‘‘abortion,’’ and ‘‘slippery slope,’’ is the very language Alice Park works to dispel some of the ‘‘belief-as-fact’’ pattern introduced and perpetuated by those who have no greater that has driven the social side of stem cell research in her book, interest in the field than to see its demise. -

Scientists Turn Skin Cells Into Motor Neurons in ALS Patients
Scientists Turn Skin Cells Into Motor Neurons in ALS Patients By Amanda Gardner HealthDay Reporter Thursday, July 31, 2008; 12:00 AM THURSDAY, July 31 (HealthDay News) -- Scientists have turned skin cells from patients with Lou Gehrig's disease into motor neurons that are genetically identical to the patients' own neurons. An unlimited number of these neurons can now be created and studied in the laboratory, a capability which should result in a better understanding of the disease and, one day, lead to new treatments or even the production of healthy cells that can replace the diseased ones. "The hope of some scientists is that they might be able to harness stem cells and program them to generate pluripotent stem cell lines [capable of differentiating into many different types of cells] which have the genes of patients," said Kevin Eggan, co-author of a paper appearing July 31 in the online version ofScience. "This would open up the possibility of producing a large supply of immune-matched cells to that patient that could be used in transplantation methodologies." "The other hope, and one that's much closer upon us . is if you could produce the cell types that become sick in that person, you might be able to use them in the laboratory to come to understand basic aspects of the disease and take the study of disease out of patients, where it's very difficult, and put it into the Petri dish," added Eggan, who is a principal faculty member at the Harvard Stem Cell Institute and spoke about the research at a teleconference Wednesday. -
Supporting Information
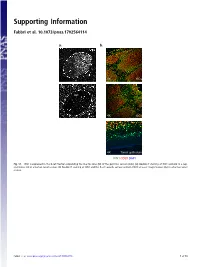
Supporting Information Fabbri et al. 10.1073/pnas.1702564114 a. b. GC 4X GCs 20x GC 4X GCs 4X Tonsil epithelium ICN1 CD20 DAPI Fig. S1. ICN1 is expressed in the B-cell fraction populating the mantle zone (M) of the germinal centers (GCs). (A) Double IF staining of ICN1 and AID in a rep- resentative GC in a human tonsil section. (B) Double IF staining of ICN1 and the B-cell–specific surface antigen CD20 at lower magnification (4×) in a human tonsil section. Fabbri et al. www.pnas.org/cgi/content/short/1702564114 1of10 Fig. S2. ICN1 expression analysis in a panel of primary CLL cases and PBMC. (A) IB analysis of ICN1 and control β-actin in a panel of 124 CLL PB primary CLL cases, (B) in primary NOTCH1–wild-type CLL cells treated with the γ-secretase inhibitor Compound E (CpE, 500 nM, 8 h) or control DMSO, and (C) in PBMC protein extracts and representative primary CLL cases expressing ICN1. Samples are color-coded based on the NOTCH1 mutational status [red, clonal NOTCH1 PEST-truncating events; orange, subclonal NOTCH1 PEST-truncating events; blue, RAG-mediated NOTCH1 translocation (83); and black, NOTCH1–wild-type]. Samples in gray were excluded from the analysis because of low quality of the protein lysate, low viability, or low leukemic representation. Color-coded arrows indicate cases subjected to RNA-Seq analysis: dark red denotes NOTCH1-mutated cases expressing ICN1; blue, NOTCH1–wild-type cases expressing ICN1; and green, ICN1− NOTCH1–wild-type cases. Abbreviations: MO+DL1, MO1043 cells cocultured on OP9-DL1 cells (54); s.e., short exposure; l.e., long exposure. -

Reopening Avenues for Attacking Amyotrophic Lateral Sclerosis 15 July 2016, by Hannah L
Reopening avenues for attacking amyotrophic lateral sclerosis 15 July 2016, by Hannah L. Robbins plays an important role in not only the nervous system, but also the blood and immune systems. "The point of our paper was to determine the function of this gene and what it normally helps to do in the body," said lead author Kevin Eggan, a professor in Harvard's Department of Stem Cell and Regenerative Biology (HSCRB) and an HSCI principal faculty member who has been studying ALS for more than a decade. According to Eggan, one way to better understand the gene is to catalog what is missing or goes awry when it is "knocked out," or inactivated. The scientists found that mice without a functional copy of the gene C9ORF72 had abnormally large spleens, livers, and lymph nodes, and got sick and In the murine spleen, lymphoid tissue (purple) is died. Mice with one working copy experienced responsible for launching an immune response to blood- similar but less severe changes. born antigens, while red pulp (pink) filters the blood. Mutations in the C9ORF72 gene, the most common mutation found in ALS patients, can inflame lymphoid "I realized immediately that the mouse knockout tissue and contribute to immune system dysfunction. had immune dysfunction," said Leonard Zon, a Credit: Dan Mordes, Eggan lab, Harvard Stem Cell professor in HSCRB, after seeing the preliminary Institute data at an informal presentation. The research team predicted that the gene mutation would affect neurons, but the finding that it Harvard Stem Cell Institute (HSCI) researchers at also inflamed other cells, namely those involved in Harvard University and the Broad Institute of autoimmunity, was "unexpected." With input from Harvard and MIT have found evidence that bone Zon and immunologist Luigi Notarangelo of Boston marrow transplantation may one day be beneficial Children's Hospital, the team decided to change to a subset of patients suffering from amyotrophic direction and further explore the gene's impact on lateral sclerosis (ALS), a fatal neurodegenerative the immune system. -

IBRAHIM ALDEAILEJ Phd.2013.Pdf
Bangor University DOCTOR OF PHILOSOPHY Identification and functional characterisation of germ line genes in human cancer cells Aldeailej, Ibrahim Award date: 2013 Awarding institution: Bangor University Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 09. Oct. 2021 Bangor University School of Biological Sciences Identification and functional characterisation of germ line genes in human cancer cells Ph.D. Thesis 2013 IBRAHIM ALDEAILEJ Declaration and Consent Details of the Work I hereby agree to deposit the following item in the digital repository maintained by Bangor University and/or in any other repository authorized for use by Bangor University. Author Name: IBRAHIM MOHAMMED ALDEAILEJ Title: Identification and functional characterisation of germ line genes in human cancer cells Supervisor/Department: Dr. Ramsay James McFarlane/ School of Biological Sciences Funding body (if any): kingdom of Saudi Arabia Government Qualification/Degree obtained: Ph.D. -

Unique Unique
UNIQUE UNIQUE (ju^'ni^k) A. adj. 1. Of which there is only one; one and no other; single, sole, solitary. 2. a. That is or forms the only one of its kind; having no like or equal; standing alone in comparison with others, freq. by reason of superior excellence; unequalled, unparalleled, unrivalled. Oxford English Dictionary HSCI is a unique scientific enterprise THE HARVARD STEM CELL INSTITUTE is a unique scientific enterprise; nowhere else in the world are as many leading scientists gathered together to specifically focus on what today is one of the most important basic question in the life sciences: What is the property that allows stem cells not only to differentiate into any cell type in the body, but also makes it possible for them to reprogram other cells? And perhaps as significant, the Harvard Stem Cell Institute is a unique scientific enterprise because it is dedicated to bringing the answers to these questions to patients’ bedsides in the form of new treatments for conditions such as Parkinson’s disease, heart disease, cancer, blindness, and even dementias. scientific enterprise 4 } For a relatively brief interval ... researchers are Researchers believe that in as little as a decade it research solely on an individual lab basis, HSCI has hand, HSCI has established two major disease In 2005 HSCI provided its first dozen seed grants, intoxicated with a mix of the newly discovered may be possible to use embryonic stem cells to established collaborative platforms with other programs, the Cancer Stem Cell Program and The totaling $1.8 million, to launch innovative work by and the imaginable unknown. -

Scientists Report a Breakthrough in Stem Cell Production Created Cells That Match Those in ALS Patient
THIS STORY HAS BEEN FORMATTED FOR EASY PRINTING Scientists report a breakthrough in stem cell production Created cells that match those in ALS patient By Carolyn Y. Johnson, Globe Staff | August 1, 2008 Reaching a milestone in stem cell research, scientists at Harvard and Columbia universities reported yesterday that they created the first stem cell lines from a sick person, then coaxed these cells to become nerve cells genetically matched to those that had gone bad in a patient's spinal cord. In a paper published online in the journal Science, the team claimed success at what researchers have long been racing to do: create in the laboratory a plentiful supply of cells that have the same genetic makeup as a patient with a particular disease. The work was done with patients suffering from ALS, or Lou Gehrig's disease, but the researchers said the same technique can be used to study many other genetic diseases. By comparing diseased cells to normal cells in a Petri dish, scientists hope to better understand what causes disease and test new drugs. A series of dramatic discoveries over the past two years has pushed stem cell science forward, so this advance was expected. Still, the scientists were thrilled to have accomplished a task that was a fundamental reason for doing stem cell research in the first place. "Since the cloning of Dolly the sheep and the first derivation of a human embryonic stem cell line by Jamie Thomson some 10 years ago, it's been the hope of scientists . to generate stem cell lines that have the genes of a patient," said Kevin Eggan, coauthor of the paper and a principal faculty member of the Harvard Stem Cell Institute. -

Overexpression of the Proneural Transcription Factor ASCL1 In
Malli et al. Molecular Cytogenetics (2018) 11:3 DOI 10.1186/s13039-018-0355-7 CASE REPORT Open Access Overexpression of the proneural transcription factor ASCL1 in chronic lymphocytic leukemia with a t(12;14)(q23.2;q32.3) Theodora Malli1, Melanie Rammer1, Sabrina Haslinger1, Jonathan Burghofer1, Sonja Burgstaller2, Hans-Christian Boesmueller3,4, Renate Marschon1, Wolfgang Kranewitter1, Martin Erdel1, Sabine Deutschbauer1 and Gerald Webersinke1* Abstract Background: Translocations of the IGH locus on 14q32.3 are present in about 8% of patients with chronic lymphocytic leukemia (CLL) and contribute to leukemogenesis by deregulating the expression of the IGH-partner genes. Identification of these genes and investigation of the downstream effects of their deregulation can reveal disease-causing mechanisms. Case presentation: We report on the molecular characterization of a novel t(12;14)(q23.2;q32.3) in CLL. As a consequence of the rearrangement ASCL1 was brought into proximity of the IGHJ-Cμ enhancer and was highly overexpressed in the aberrant B-cells of the patient, as shown by qPCR and immunohistochemistry. ASCL1 encodes for a transcription factor acting as a master regulator of neurogenesis, is overexpressed in neuroendocrine tumors and a promising therapeutic target in small cell lung cancer (SCLC). Its overexpression has also been recently reported in acute adult T-cell leukemia/lymphoma. To examine possible downstream effects of the ASCL1 upregulation in CLL, we compared the gene expression of sorted CD5+ cells of the translocation patient to that of CD19+ B-cells from seven healthy donors and detected 176 significantly deregulated genes (Fold Change ≥2, FDR p ≤ 0.01). -

The Effect of Prpsc Accumulation on Inflammatory Gene
Edinburgh Research Explorer The effect of PrP(Sc) accumulation on inflammatory gene expression within sheep peripheral lymphoid tissue Citation for published version: Gossner, AG & Hopkins, J 2015, 'The effect of PrP(Sc) accumulation on inflammatory gene expression within sheep peripheral lymphoid tissue', Veterinary Microbiology, vol. 181, no. 3-4, pp. 204-211. https://doi.org/10.1016/j.vetmic.2015.10.013 Digital Object Identifier (DOI): 10.1016/j.vetmic.2015.10.013 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Veterinary Microbiology General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 29. Sep. 2021 G Model VETMIC 7118 No. of Pages 8 Veterinary Microbiology xxx (2015) xxx–xxx Contents lists available at ScienceDirect Veterinary Microbiology journal homepage: www.elsevier.com/locate/vetmic Sc The effect of PrP accumulation on inflammatory gene expression within sheep peripheral lymphoid tissue Anton G. Gossner, John Hopkins* The Roslin Institute & R(D)SVS, University of Edinburgh, Easter Bush, Midlothian EH25 9RG, UK A R T I C L E I N F O A B S T R A C T Sc Article history: Accumulation of the misfolded prion protein, PrP in the central nervous system (CNS) is strongly linked Received 27 April 2015 to progressive neurodegenerative disease. -

Analysis of Human Embryos from Zygote to Blastocyst Reveals Distinct Gene Expression Patterns Relative to the Mouse
Developmental Biology 375 (2013) 54–64 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse Kathy K. Niakan a,b,n, Kevin Eggan a,nn a The Howard Hughes Medical Institute, Harvard Stem Cell Institute and the Department of Stem Cell and Regenerative Biology, Harvard University, 7 Divinity Avenue, Cambridge, MA 02138, USA b Centre for Trophoblast Research, Department of Physiology, Development and Neuroscience, Anne McLaren Laboratory for Regenerative Medicine, University of Cambridge, West Forvie Building, Robinson Way, Cambridge CB2 0SZ, UK article info abstract Article history: Early mammalian embryogenesis is controlled by mechanisms governing the balance between Received 21 July 2012 pluripotency and differentiation. The expression of early lineage-specific genes can vary significantly Received in revised form between species, with implications for developmental control and stem cell derivation. However, the 29 November 2012 mechanisms involved in patterning the human embryo are still unclear. We analyzed the appearance Accepted 11 December 2012 and localization of lineage-specific transcription factors in staged preimplantation human embryos Available online 19 December 2012 from the zygote until the blastocyst. We observed that the pluripotency-associated transcription Keywords: factor OCT4 was initially expressed in 8-cell embryos at 3 days post-fertilization (dpf), and restricted to Human embryo the inner cell mass (ICM) in 128–256 cell blastocysts (6 dpf), approximately 2 days later than the Human embryonic stem cell derivation mouse. The trophectoderm (TE)-associated transcription factor CDX2 was upregulated in 5 dpf Primitive endoderm blastocysts and initially coincident with OCT4, indicating a lag in CDX2 initiation in the TE lineage, Epiblast progenitor Trophectoderm relative to the mouse. -

Stem-Cell Researchers Claim Embryo Labs Are Still a Necessity
Stem-Cell Researchers Claim Embryo Labs Are Still a Necessity January 4, 2008 The surveillance cameras and electronic locks are the only hints that a visitor has reached the border of a scientific frontier. Behind these laboratory doors a few blocks from Columbia University in Manhattan is a research enterprise quarantined by federal law because, for many people, it poses a moral hazard. A nonprofit foundation called Project ALS set up this privately funded lab -- the Jennifer Estess Laboratory for Stem Cell Research -- 18 months ago to allow scientists to circumvent federal restrictions on experiments with cells made from human embryos, often left over from commercial in vitro fertilization. Within its walls, researchers from Columbia, Harvard Medical School, the Salk Institute and others are studying embryonic cells in an effort to overcome an incurable fatal disease called amyotrophic lateral sclerosis, also known as Lou Gehrig's disease. Under extreme magnification, motor neurons derived from human embryonic stem cells reveal their structure. All nuclei are shown in blue; all neurons shown in red; nuclear proteins specific to motor neuron are shown in green and purple. The stem cells from which the neurons developed were made with human embryos created during in vitro fertilization treatment. The Estess lab is a modest outpost in a network of new state and private stem-cell biology labs that together command billions of dollars in nonfederal funds for research into how embryonic cells might one day reverse ALS and other conditions, such as leukemia, diabetes and Parkinson's disease. In November, stem-cell researchers in Japan and the U.S. -

Ep 2885427 B1
(19) TZZ _T (11) EP 2 885 427 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C12Q 1/68 (2018.01) 10.01.2018 Bulletin 2018/02 (86) International application number: (21) Application number: 13759978.3 PCT/EP2013/002462 (22) Date of filing: 14.08.2013 (87) International publication number: WO 2014/026768 (20.02.2014 Gazette 2014/08) (54) COLORECTAL CANCER METHYLATION MARKER KOLOREKTALKREBSMETHYLIERUNGSMARKER MARQUEUR DE METHYLATION POUR LE CANCER COLORECTAL (84) Designated Contracting States: • RAFAEL A IRIZARRY ET AL: "The human colon AL AT BE BG CH CY CZ DE DK EE ES FI FR GB cancer methylome shows similar hypo- and GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO hypermethylation at conserved tissue-specific PL PT RO RS SE SI SK SM TR CpG island shores", NATURE GENETICS, vol. 41, no. 2, 1 February 2009 (2009-02-01), pages (30) Priority: 14.08.2012 EP 12180459 178-186, XP055029485, ISSN: 1061-4036, DOI: 10.1038/ng.298 (43) Date of publication of application: • M. D. ROBINSON ET AL: "Evaluation of 24.06.2015 Bulletin 2015/26 affinity-based genome-wide DNA methylation data: Effects of CpG density, amplification bias, (73) Proprietor: Max-Planck-Gesellschaft zur and copy number variation", GENOME Förderung RESEARCH, vol. 20, no. 12, 1 December 2010 der Wissenschaften (2010-12-01), pages 1719-1729, XP055041625, 80539 Munich (DE) ISSN: 1088-9051, DOI: 10.1101/gr.110601.110 • E. A. HOUSEMAN ET AL: "Copy number variation (72) Inventors: has little impact on bead-array-based measures • SCHWEIGER, Michal-Ruth of DNA methylation", BIOINFORMATICS, vol.