Sentinel Lymph Node Biopsy for Invasive Breast Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Follicular Lymphoma
Follicular Lymphoma What is follicular lymphoma? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines FOLLICULAR LYMPHOMA: A GUIDE FOR PATIENTS PATIENT INFORMATION BASED ON ESMO CLINICAL PRACTICE GUIDELINES This guide for patients has been prepared by the Anticancer Fund as a service to patients, to help patients and their relatives better understand the nature of follicular lymphoma and appreciate the best treatment choices available according to the subtype of follicular lymphoma. We recommend that patients ask their doctors about what tests or types of treatments are needed for their type and stage of disease. The medical information described in this document is based on the clinical practice guidelines of the European Society for Medical Oncology (ESMO) for the management of newly diagnosed and relapsed follicular lymphoma. This guide for patients has been produced in collaboration with ESMO and is disseminated with the permission of ESMO. It has been written by a medical doctor and reviewed by two oncologists from ESMO including the lead author of the clinical practice guidelines for professionals, as well as two oncology nurses from the European Oncology Nursing Society (EONS). It has also been reviewed by patient representatives from ESMO’s Cancer Patient Working Group. More information about the Anticancer Fund: www.anticancerfund.org More information about the European Society for Medical Oncology: www.esmo.org For words marked with an asterisk, a definition is provided at the end of the document. Follicular Lymphoma: a guide for patients - Information based on ESMO Clinical Practice Guidelines – v.2014.1 Page 1 This document is provided by the Anticancer Fund with the permission of ESMO. -

Updates in Assessment of the Breast After Neoadjuvant Treatment
Updates in Assessment of The Breast After Neoadjuvant Treatment Laila Khazai 3/3/18 AJCC, 8th Edition AJCC • Pathologic Prognostic Stage is not applicable for patients who receive neoadjuvant therapy. • Pathologic staging includes all data used for clinical staging, plus data from surgical resection. • Information recorded should include: – Clinical Prognostic Stage. – The category information for either clinical (ycT and ycN) response to therapy if surgery is not performed, or pathologic (ypT and ypN) if surgery is performed. – Degree of response (complete, partial, none). AJCC • Post -treatment size should be estimated based on the best combination of imaging, gross, and microscopic histological findings. • The ypT is determined by measuring the largest single focus of residual invasive tumor, with a modifier (m) indicating multiple foci of residual tumor. • This measurement does not include areas of fibrosis within the tumor bed. • When the only residual cancer intravascular or intralymphatic (LVI), the yPT0 category is assigned, but it is not classified as complete pathologic response. A formal system (i.e. RCB, Miller-Payne, Chevalier, …) may be offered in the report. Otherwise, description of the distance over which tumor foci extend, the number of tumor foci present, or the number of tumor slides/blocks in which tumor appears might be offered. AJCC • The ypN categories are the same as those used for pN. • Only the largest contiguous focus of residual tumor is used for classification (treatment associated fibrosis is not included). • Inclusion of additional information such as distance over which tumor foci extend and the number of tumor foci present, may assist the clinician in estimating the extent of residual disease. -

The Updated AJCC/TNM Staging System (8Th Edition) for Oral Tongue Cancer
166 Editorial The updated AJCC/TNM staging system (8th edition) for oral tongue cancer Kyubo Kim, Dong Jin Lee Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University College of Medicine, Seoul, South Korea Correspondence to: Dong Jin Lee, MD, PhD. 1 Singil-ro, Yeongdeungpo-gu, Seoul 150-950, South Korea. Email: [email protected]. Comment on: Almangush A, Mäkitie AA, Mäkinen LK, et al. Small oral tongue cancers (≤ 4 cm in diameter) with clinically negative neck: from the 7th to the 8th edition of the American Joint Committee on Cancer. Virchows Arch 2018;473:481-7. Submitted Dec 22, 2018. Accepted for publication Dec 28, 2018. doi: 10.21037/tcr.2019.01.02 View this article at: http://dx.doi.org/10.21037/tcr.2019.01.02 An increasing amount of literature shows solid evidence that updated classification system and the applicability of DOI as the depth of invasion (DOI) of oral cavity squamous cell a predictor of clinical behavior for early-stage OTSCC. carcinoma is an independent predictor for occult metastasis, The AJCC 8th edition employs a cut-off value of 5 mm recurrence, and survival (1-3). Furthermore, the DOI of the DOI for upstaging from stage T1 to T2 and 10 mm for primary tumor has been a major criterion when deciding to upstaging to T3. This may be questionable as it has been perform elective neck dissection on oral cavity squamous shown that an invasion depth of more than 4 mm increases cell carcinoma patients since as early as the mid-1990s (4). the risk of locoregional metastasis and is associated with a A cut-off value of 4 mm has conventionally been used poor prognosis (9-11), but with the new staging system, a when determining the need for elective neck dissection, large number of invasive tumors in which the DOI is less based on a study by Kligerman et al. -

Oncology 101 Dictionary
ONCOLOGY 101 DICTIONARY ACUTE: Symptoms or signs that begin and worsen quickly; not chronic. Example: James experienced acute vomiting after receiving his cancer treatments. ADENOCARCINOMA: Cancer that begins in glandular (secretory) cells. Glandular cells are found in tissue that lines certain internal organs and makes and releases substances in the body, such as mucus, digestive juices, or other fluids. Most cancers of the breast, pancreas, lung, prostate, and colon are adenocarcinomas. Example: The vast majority of rectal cancers are adenocarcinomas. ADENOMA: A tumor that is not cancer. It starts in gland-like cells of the epithelial tissue (thin layer of tissue that covers organs, glands, and other structures within the body). Example: Liver adenomas are rare but can be a cause of abdominal pain. ADJUVANT: Additional cancer treatment given after the primary treatment to lower the risk that the cancer will come back. Adjuvant therapy may include chemotherapy, radiation therapy, hormone therapy, targeted therapy, or biological therapy. Example: The decision to use adjuvant therapy often depends on cancer staging at diagnosis and risk factors of recurrence. BENIGN: Not cancerous. Benign tumors may grow larger but do not spread to other parts of the body. Also called nonmalignant. Example: Mary was relieved when her doctor said the mole on her skin was benign and did not require any further intervention. BIOMARKER TESTING: A group of tests that may be ordered to look for genetic alterations for which there are specific therapies available. The test results may identify certain cancer cells that can be treated with targeted therapies. May also be referred to as genetic testing, molecular testing, molecular profiling, or mutation testing. -

Sentinel Lymph Node Mapping in Endometrial Cancer: a Comprehensive Review
REVIEW published: 29 June 2021 doi: 10.3389/fonc.2021.701758 Sentinel Lymph Node Mapping in Endometrial Cancer: A Comprehensive Review Lirong Zhai 1, Xiwen Zhang 2, Manhua Cui 2 and Jianliu Wang 1* 1 Department of Gynecology and Obstetrics, Peking University People’s Hospital, Beijing, China, 2 Department of Gynecology and Obstetrics, The Second Hospital of Jilin University, Changchun, China Endometrial cancer (EC) is known as a common gynecological malignancy. The incidence rate is on the increase annually. Lymph node status plays a crucial role in evaluating the prognosis and selecting adjuvant therapy. Currently, the patients with high-risk (not comply with any of the following: (1) well-differentiated or moderately differentiated, pathological grade G1 or G2; (2) myometrial invasion< 1/2; (3) tumor diameter < 2 cm are commonly recommended for a systematic lymphadenectomy (LAD). However, conventional LAD shows high complication incidence and uncertain survival benefits. Sentinel lymph node (SLN) refers to the first lymph node that is passed by the lymphatic Edited by: metastasis of the primary malignant tumor through the regional lymphatic drainage Fabio Martinelli, pathway and can indicate the involvement of lymph nodes across the drainage area. Istituto Nazionale dei Tumori (IRCCS), Italy Mounting evidence has demonstrated a high detection rate (DR), sensitivity, and negative Reviewed by: predictive value (NPV) in patients with early-stage lower risk EC using sentinel lymph node Benedetta Guani, mapping (SLNM) with pathologic ultra-staging. Meanwhile, SLNM did not compromise the Centre Hospitalier Universitaire ’ Vaudois (CHUV), Switzerland patient s progression-free survival (PFS) and overall survival (OS) with low operative Giulio Sozzi, complications. -

Cancer Treatment and Survivorship Facts & Figures 2019-2021
Cancer Treatment & Survivorship Facts & Figures 2019-2021 Estimated Numbers of Cancer Survivors by State as of January 1, 2019 WA 386,540 NH MT VT 84,080 ME ND 95,540 59,970 38,430 34,360 OR MN 213,620 300,980 MA ID 434,230 77,860 SD WI NY 42,810 313,370 1,105,550 WY MI 33,310 RI 570,760 67,900 IA PA NE CT 243,410 NV 185,720 771,120 108,500 OH 132,950 NJ 543,190 UT IL IN 581,350 115,840 651,810 296,940 DE 55,460 CA CO WV 225,470 1,888,480 KS 117,070 VA MO MD 275,420 151,950 408,060 300,200 KY 254,780 DC 18,750 NC TN 470,120 AZ OK 326,530 NM 207,260 AR 392,530 111,620 SC 143,320 280,890 GA AL MS 446,900 135,260 244,320 TX 1,140,170 LA 232,100 AK 36,550 FL 1,482,090 US 16,920,370 HI 84,960 States estimates do not sum to US total due to rounding. Source: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Contents Introduction 1 Long-term Survivorship 24 Who Are Cancer Survivors? 1 Quality of Life 24 How Many People Have a History of Cancer? 2 Financial Hardship among Cancer Survivors 26 Cancer Treatment and Common Side Effects 4 Regaining and Improving Health through Healthy Behaviors 26 Cancer Survival and Access to Care 5 Concerns of Caregivers and Families 28 Selected Cancers 6 The Future of Cancer Survivorship in Breast (Female) 6 the United States 28 Cancers in Children and Adolescents 9 The American Cancer Society 30 Colon and Rectum 10 How the American Cancer Society Saves Lives 30 Leukemia and Lymphoma 12 Research 34 Lung and Bronchus 15 Advocacy 34 Melanoma of the Skin 16 Prostate 16 Sources of Statistics 36 Testis 17 References 37 Thyroid 19 Acknowledgments 45 Urinary Bladder 19 Uterine Corpus 21 Navigating the Cancer Experience: Treatment and Supportive Care 22 Making Decisions about Cancer Care 22 Cancer Rehabilitation 22 Psychosocial Care 23 Palliative Care 23 Transitioning to Long-term Survivorship 23 This publication attempts to summarize current scientific information about Global Headquarters: American Cancer Society Inc. -

December 2020 E-Tips Solid Tumor Rules
New Jersey State Cancer Registry December 2020 E-Tips Cancer Epidemiology Services http://www.nj.gov/health/ces (609) 633-0500 Solid Tumor Rules: December 2020 Update ICD-0-3.2 changes have also been added to applicable site modules. Most changes are minor: terminology, additional definitions, new notes and examples. In order to clarify histology coding instructions, new rules have been added and histology tables updated. These updates do not require review of already abstracted cases. The December 2020 rules replace the current rules and should be used now. SEER Strongly recommends reading the December 2020 Change Log to understand what changes were made. The updated Solid Tumor Rules may be accessed at: https://seer.cancer.gov/tools/solidtumor/ Reportability Changes for 2021 Radiation Coding Total Dose (1533) Starting 01/01/2021 the following terms are reportable: If doses across phases to a single point of region, code Sum of all phases. ** (see 2019 update below) Early or evolving melanoma in situ, or any other early or If doses are to multiple metastatic sites, code highest evolving melanoma, are reportable. dose site. If doses are to primary site and metastatic site, code dose All GIST tumors are reportable and classified as 8936/3 in from the primary site only. ICD-O-3.2. When you have two different sites, you cannot add the Nearly all thymomas are reportable; the exceptions are doses together to get the total dose. microscopic thymoma or thymoma benign (8580/0), micronodular thymoma with lymphoid stroma (8580/1), Radiation Tips and updates for 2019 and ectopic hamartomatous thymoma (8587/0). -

About Mastectomy and Sentinel Lymph Node Biopsy
All About Mastectomy and Sentinel Lymph Node Biopsy One of the most important goals of Moffitt Cancer Center is to provide you with quality patient care through education, research and patient care. The following information has been developed to help you understand the mastectomy and sentinel lymph node biopsy procedures for which you have been scheduled. Members of your health care team will review this information with you and answer any questions that you may have. Definitions: Mastectomy – Removal of the entire breast but not the muscles underneath. Lymph Nodes - Small bean shaped glands found in the armpit. They remove waste and fluids from the arm and breast and help fight infection. They are also call lymph glands. Sentinel Lymph Node - The first place breast cancer may metastasize (or spread) is to lymph nodes in the axilla (underarm area) on the side of the body where the cancer is. The first lymph node(s) are referred to as the sentinel lymph node(s) or the “gate keepers”. These nodes are first biopsied to determine if the cancer has spread. If cancer cells are not found in the sentinel lymph node(s) that were removed, it will not be necessary to remove the remaining lymph nodes in the arm pit. Sentinel Lymph Node Mapping and Biopsy - This procedure is a two step process. Performing the sentinel lymph node biopsy requires a small incision in your axilla. In the first step, the doctor injects a radioactive substance and/ or blue dye in the area around the tumor. Lymphatic channels (like blood vessels) carry these materials to the sentinel lymph node. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

Application of Preoperative Computed Tomographic Lymphography For
Wen et al. BMC Surg (2021) 21:187 https://doi.org/10.1186/s12893-021-01190-7 RESEARCH ARTICLE Open Access Application of preoperative computed tomographic lymphography for precise sentinel lymph node biopsy in breast cancer patients Shishuai Wen1,2,3†, Yiran Liang1†, Xiaoli Kong1, Baofeng Liu4, Tingting Ma1, Yeqing Zhou1, Liyu Jiang1, Xiaoyan Li1 and Qifeng Yang1,5,6* Abstract Background: In light of the extensive application of sentinel lymph node biopsy (SLNB) in clinically node-negative breast cancer patients and the recently investigated failure of SLNB after lumpectomy, it has become important to explore methods for preoperative mapping of sentinel lymph nodes (SLNs) and their lymphatics to direct precise SLNB and improve the identifcation rate of SLNs. Methods: Twenty-seven patients with suspected breast cancer based on the results of the clinical examination and imaging were enrolled in the study. Computed tomographic lymphography (CTLG) followed by CT three-dimensional reconstruction was performed to determine the localization of SLNs and lymphatics on the body surface preopera- tively. Intraoperatively combined staining with methylene blue and indocyanine green was used to evaluate the accuracy and feasibility of CTLG. Results: SLNs and lymphatics from the breast were identifed using CTLG in all patients, and preoperative SLNs and lymphatics localization on the body surface showed a signifcant role in the selection of operative incision and injec- tion points. The accuracy rate of SLN and lymphatic detection by CTLG was 92.6% compared with intraoperatively combined staining. Moreover, preoperative CTLG performed well in SLN number detection, and the accuracy rate was 95.2%. Conclusion: We evaluate the procedure and application of preoperative CTLG in the superfcial localization of SLNs and lymphatics, which may lead to a decreased incidence of cutting of the lymphatics of SLNs and consequently more rapid and accurate SLN detection. -

Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma
Journal of Clinical Medicine Review Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma Ane Gerda Z Eriksson 1,2,* , Ben Davidson 2,3, Pernille Bjerre Trent 1,2, Brynhildur Eyjólfsdóttir 1, Gunn Fallås Dahl 1, Yun Wang 1 and Anne Cathrine Staff 2,4 1 Department of Gynecologic Oncology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway; [email protected] (P.B.T.); [email protected] (B.E.); [email protected] (G.F.D.); [email protected] (Y.W.) 2 Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, N-0316 Oslo, Norway; [email protected] (B.D.); [email protected] (A.C.S.) 3 Department of Pathology, Oslo University Hospital, Norwegian Radium Hospital, N-0310 Oslo, Norway 4 Division of Obstetrics and Gynaecology, Oslo University Hospital, Ullevål, N-0424 Oslo, Norway * Correspondence: [email protected] Abstract: Sentinel lymph node (SLN) biopsy has emerged as an alternative staging approach in women with assumed early-stage endometrial carcinoma. Through image-guided surgery and pathologic ultrastaging, the SLN approach is introducing “precision medicine” to the surgical management of gynecologic cancers, providing a comprehensive evaluation of high-yield lymph nodes. This approach improves the surgeons’ ability to detect small-volume metastatic disease while reducing intraoperative and postoperative morbidity associated with lymphadenectomy. Although the majority of clinicians in Europe and the USA have recognized the value of SLN biopsy in endometrial carcinoma and introduced this as part of clinical practice, there is ongoing debate Citation: Eriksson, A.G.Z; Davidson, regarding its role in very low-risk patients as well as in patients at high risk of nodal metastasis. -
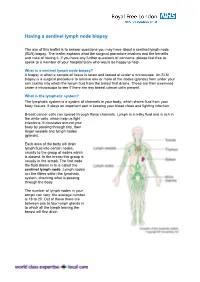
Having a Sentinel Lymph Node Biopsy
Having a sentinel lymph node biopsy The aim of this leaflet is to answer questions you may have about a sentinel lymph node (SLN) biopsy. The leaflet explains what the surgical procedure involves and the benefits and risks of having it. If you have any further questions or concerns, please feel free to speak to a member of your hospital team who would be happy to help. What is a sentinel lymph node biopsy? A biopsy is when a sample of tissue is taken and looked at under a microscope. An SLN biopsy is a surgical procedure to remove one or more of the nodes (glands) from under your arm (axilla) into which the lymph fluid from the breast first drains. These are then examined under a microscope to see if there are any breast cancer cells present. What is the lymphatic system? The lymphatic system is a system of channels in your body, which drains fluid from your body tissues. It plays an important part in keeping your blood clean and fighting infection. Breast cancer cells can spread through these channels. Lymph is a milky fluid and is rich in the white cells, which help us fight infections. It circulates around your body by passing through tiny, then larger vessels and lymph nodes (glands). Each area of the body will drain lymph fluid into certain nodes, usually to the group of nodes which is closest. In the breast this group is usually in the armpit. The first node the fluid drains in to is called the sentinel lymph node.