Hypervalent Surface Interactions for Colloidal Stability and Doping of Silicon Nanocrystals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicological Profile for 2-Butanone Released for Public Comment in May 2019
Toxicological Profile for 2-Butanone October 2020 2-BUTANONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. 2-BUTANONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -

Hydroxyethoxyphenyl Butanone’ (HEPB)
SCCS/1582/16 Final version Scientific Committee on Consumer Safety SCCS OPINION ON Ethylzingerone – ‘Hydroxyethoxyphenyl Butanone’ (HEPB) (Cosmetics Europe No P98) The SCCS adopted this Opinion by written procedure on 7 April 2017 SCCS/1582/16 Final Opinion on Ethylzingerone - ‘Hydroxyethoxyphenyl Butanone’ (HEPB) - Cosmetics Europe No P98 About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide Opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Ulrike Bernauer, Laurent Bodin, Leonardo Celleno, Qasim Chaudhry, Pieter Jan Coenraads, Maria Dusinska, Janine Ezendam, Eric Gaffet, Corrado Lodovico Galli, Berit Granum, Eirini Panteri, Vera Rogiers, Christophe Rousselle, Maciej Stepnik, Tamara Vanhaecke, Susan Wijnhoven. -

Critical Evaluation of Thermochemical Properties of C1–C4 Species; Updated Group-Contributions to Estimate Thermochemical Properties
Provided by the author(s) and NUI Galway in accordance with publisher policies. Please cite the published version when available. Critical evaluation of thermochemical properties of C-1-C-4 Title species: updated group-contributions to estimate thermochemical properties Author(s) Burke, S. M.; Simmie, J. M.; Curran, Henry J. Publication Date 2015-01-05 Burke, S. M., Simmie, J. M., & Curran, H. J. (2015). Critical Publication evaluation of thermochemical properties of C-1-C-4 species: Information updated group-contributions to estimate thermochemical properties. Journal of Physical and Chemical Reference Data, 44(1), 013101. Publisher AIP Publishing Link to publisher's http://dx.doi.org/10.1063/1.4902535 version Item record http://hdl.handle.net/10379/6119 DOI http://dx.doi.org/10.1063/1.4902535 Downloaded 2021-09-25T06:51:18Z Some rights reserved. For more information, please see the item record link above. C1–C4 thermochemical properties: updated group additivity groups Critical evaluation of thermochemical properties of C1–C4 species; updated group-contributions to estimate thermochemical properties. S. M. Burke, J. M. Simmie, and H. J. Currana) Combustion Chemistry Centre, National University of Ireland, Galway, Ireland (Dated: 26 September 2014) A review of literature enthalpies of formation and molar entropies for alkanes, alkenes, alcohols, hydroperox- ides, and their associated radicals has been compiled and critically evaluated. By comparing literature values the overall uncertainty in thermochemical properties of small hydrocarbons and oxygenated hydrocarbons can be highlighted. In general there is good agreement between heat of formation values in the literature for stable species, however there is greater uncertainty in the values for radical species and for molar entropy values. -

The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”
chemengineering Article Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy” Robert J. Meier Pro-Deo Consultant, 52525 Heinsberg, North-Rhine Westphalia, Germany; [email protected] Abstract: Group contribution (GC) methods to predict thermochemical properties are of eminent importance to process design. Compared to previous works, we present an improved group contri- bution parametrization for the heat of formation of organic molecules exhibiting chemical accuracy, i.e., a maximum 1 kcal/mol (4.2 kJ/mol) difference between the experiment and model, while, at the same time, minimizing the number of parameters. The latter is extremely important as too many parameters lead to overfitting and, therewith, to more or less serious incorrect predictions for molecules that were not within the data set used for parametrization. Moreover, it was found to be important to explicitly account for common chemical knowledge, e.g., geminal effects or ring strain. The group-related parameters were determined step-wise: first, alkanes only, and then only one additional group in the next class of molecules. This ensures unique and optimal parameter values for each chemical group. All data will be made available, enabling other researchers to extend the set to other classes of molecules. Keywords: enthalpy of formation; thermodynamics; molecular modeling; group contribution method; quantum mechanical method; chemical accuracy; process design Citation: Meier, R.J. Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”. 1. Introduction ChemEngineering 2021, 5, 24. To understand chemical reactivity and/or chemical equilibria, knowledge of thermo- o https://doi.org/10.3390/ dynamic properties such as gas-phase standard enthalpy of formation DfH gas is a necessity. -

Supporting Information
Photocatalytic Atom Transfer Radical Addition to Olefins utilizing Novel Photocatalysts Errika Voutyritsa, Ierasia Triandafillidi, Nikolaos V. Tzouras, Nikolaos F. Nikitas, Eleftherios K. Pefkianakis, Georgios C. Vougioukalakis* and Christoforos G. Kokotos* Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece SUPPORTING INFORMATION Page General Remarks S2 Optimization of the Reaction Conditions for the Photocatalytic Reaction S3 between 1-decene and BrCH2CN Synthesis of Photocatalysts S5 Synthesis of the Starting Materials S12 General Procedure for the Photocatalytic Reaction between Olefins and S18 BrCH2CN General Procedure for the Photocatalytic Reaction between Olefins and S29 BrCCl3 Determination of the Quantum Yield S33 Phosphorescence Quenching Studies S36 References S41 NMR Spectra S43 E. Voutyritsa, I. Triandafillidi, N. V. Tzouras, N. F. Nikitas, E. K. Pefkianakis, G. C. Vougioukalakis & C. G. Kokotos S1 General Remarks Chromatographic purification of products was accomplished using forced-flow ® chromatography on Merck Kieselgel 60 F254 230-400 mesh. Thin-layer chromatography (TLC) was performed on aluminum backed silica plates (0.2 mm, 60 F254). Visualization of the developed chromatogram was performed by fluorescence quenching, using phosphomolybdic acid, anisaldehyde or potassium permanganate stains. Mass spectra (ESI) were recorded on a Finningan® Surveyor MSQ LC-MS spectrometer. HRMS spectra were recorded on Bruker® Maxis Impact QTOF spectrometer. 1H and 13C NMR spectra were recorded on Varian® Mercury (200 MHz and 50 MHz respectively), or a Bruker® Avance (500 MHz and 125 MHz), and are internally referenced to residual solvent signals. Data for 1H NMR are reported as follows: chemical shift (δ ppm), integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br s = broad singlet), coupling constant and assignment. -

Appendix D MEK Product Brochure
Appendix D MEK Product Brochure PRODUCT DESCRIPTION 1 of 2 Methyl Ethyl Ketone (2-Butanone, MEK) O ll MW = 72.11 CH3CCH2CH3 Methyl Ethyl Ketone, Chemical Abstracts Registry Number 78-93-3 Wiswesser Line Formula Chemical Notation 2V1 Methyl Ethyl Ketone (MEK) is a accounts for its largest use. Other important applications are as clear, colorless, low-boiling organ- Nitrocellulose lacquers consume an extraction solvent for the ic liquid with a typical ketone large volumes of Methyl Ethyl dewaxing of lube oil and as an odor. It is a fast evaporating sol- Ketone and solubilization of intermediate in the production of vent with an evaporation rate simi- acrylic coatings also contributes to antioxidants, perfumes and cata- lar to ethyl acetate. Although not the overall use of Methyl Ethyl lysts. Methyl Ethyl Ketone is also as volatile as acetone, Methyl Ketone by the surface coatings used by the hard wood pulping Ethyl Ketone is similar to acetone industry. MEK is preferred as a industry and in the production of in many respects. It is miscible lacquer solvent, because high con- smokeless powder. It is routinely with most organic solvents and is centrations of resins possessing used in printing inks, degreasing an excellent solvent for most natu- superior aliphatic and aromatic and cleaning fluids and as a com- ral and synthetic resins. diluent tolerance can be readily ponent of the solvent system used achieved as low viscosity solu- in producing magnetic tape. Methyl Ethyl Ketone is a highly tions. versatile organic compound but Methyl Ethyl Ketone as a chemi- finds its greatest utility as a sol- Methyl Ethyl Ketone is also com- cal intermediate will undergo the vent in the surface coatings indus- monly used as a solvent for rubber typical reactions associated with try. -
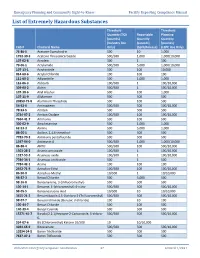
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Methyl Ethyl Ketone
Shell Chemicals Technical Datasheet Methyl Ethyl Ketone Product Code S2113 Region Global Product Category Ketones CAS Registry Number 78-93-3 Synonym(s) 2-Butanone, MEK Description Methyl ethyl ketone, MEK, a low boiling, fast evaporating solvent is a Methyl ethyl ketone, MEK, is a low boiling, fast evaporating solvent colourless, stable liquid, partially miscible with water. MEK has exceptionally good solvency for many synthetic and natural resins that are used in the formulation of printing inks, lacquers, and other types of coatings. Typical Properties Property Unit Method Value Purity, min. %m/m GC 99.5 Water %m/m ASTM D1364 0.03 Acidity (as Acetic Acid) %m/m ASTM D1613 0.002 Density at 20°C kg/l ASTM D4052 0.805 Specific Gravity at 20°C/20°C - ASTM D4052 0.806 Specific Gravity at 25°C/25°C - ASTM D4052 0.802 Coefficient of Cubic Expansion at 20°C 10-4/°C Calculated 13 Refractive Index at 20°C - ASTM D1218 1.379 Colour Pt-Co ASTM D1209 < 5 Boiling Point °C - 80 Relative Evaporation Rate (nBuAc=1) - ASTM D3539 4.0 Relative Evaporation Rate (Ether=1) - DIN 53170 3.3 Antoine Constant A # kPa. °C - 6.18444 Antoine Constant B # kPa. °C - 1259.22 Antoine Constant C # kPa. °C - 221.758 26 Methyl Ethyl Ketone March 2016 Temperature Limits for Antoine Equation # °C - -40 to +90 Vapour Pressure at 20°C kPa Calculated 9.5 Vapour Pressure at 50°C kPa Calculated 36 Saturated Vapor Concentration at 20°C g/m3 Calculated 280 Volatile Organic Compound (VOC) g/l EU / EPA 805 Flash Point (Abel) °C IP 170 -6 Auto Ignition Temperature °C ASTM E659 515 -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

How to Use This Guide Dielectric Constant Table
Dielectric Constant Table.xls Dielectric Constant Table Dielectric Constant (k) is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current. The capacitance created by the presence of the material is directly related to the Dielectric Constant of the material. Alphabetic Table A B C D Knowing the Dielectric Constant (k) of a material is needed to properly E F G H design and apply instruments such as level controls using radar, RF I J K L admittance, or capacitance technologies. There are also analytical M N O P reasons to know the (k) of a material. Q R S T U V W X How to use this guide Y Z # CLIPPER CONTROLS has compiled an extensive list of products with Dielectric Constants. Many of these Dielectric Constants are given at specific temperatures. If your product's temperature is significantly different from those listed there is a good chance that the Dielectric Constant may be different from the values listed. The products in this reference are listed in alphabetical order and are grouped in sections by the first letter of their name. Proper chemical names were used, and any trade names are the trademark of their respective owners. If you know the correct spelling of the name of the product you wish to review then use the "Search" feature on the web browser to locate the name in the list. You may also click on the letter from the alphabetical table to go directly to the beginning of that alphabetic section. -

Compilation and Analysis of Types and Concentrations of Airborne 3 Chemicals Measured in Various Indoor and Outdoor Human 4 Environments 5 6 7 8 9 1 2 10 J
Chemosphere,127,70-86,2015 1 2 Compilation and Analysis of Types and Concentrations of Airborne 3 Chemicals Measured in Various Indoor and Outdoor Human 4 Environments 5 6 7 8 9 1 2 10 J. Enrique Cometto-Muñiz and Michael H. Abraham 11 1 12 University of California, San Diego, La Jolla, California, USA and 2 13 Department of Chemistry, University College London, London, UK 14 15 16 17 18 19 20 Address for correspondence: 21 22 J. Enrique Cometto-Muñiz, Ph.D. 23 Research Scientist Emeritus, UCSD 24 8950 Villa La Jolla Drive, Suite C135 25 La Jolla, CA 92037 26 USA 27 28 29 Phone: (858) 622-5832 30 e-mail: [email protected] 31 32 33 34 35 36 37 38 39 40 Running head: Airborne Chemical Concentrations Indoors and Outdoors 41 1 41 Abstract 42 43 The main purpose of this article is to summarize and illustrate the results of a literature search on 44 the types, levels, relative concentrations, concentration spread of individual chemicals, and number of 45 airborne compounds (mostly volatile organic compounds, VOCs) that have been found, measured, and 46 reported both indoors and outdoors. Two broad categories of indoor environments are considered: 1) 47 Home/School, and 2) Commercial spaces. Also, two categories of outdoor environments are considered: 48 1) Non-industrial and 2) Industrial (the latter represented by the vicinity of a pig farm and the vicinity of an 49 oil refinery). The outcome is presented as a series of graphs and tables containing the following statistics: 50 geometric mean, arithmetic mean, median, standard deviation, variance, standard error, interquartile 51 distance, minimum value, maximum value, and number of data (data count) for the air concentration of 52 each reported compound in a given environment. -

Laboratory and Chemical Storage Areas Must Be Kept Locked When Not in Use
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ EMERGENCY TELEPHONE NUMBERS: 911 for Emergency and urgent consultation 3-2035 MSU Police; 3-2066 Facilities Management 3-2179 or (606) 207-0629 Eddie Frazier, Director of Risk Management 3-2584 or (606) 207-9425 Holly Niehoff, Environmental Health & Safety Specialist 3-2099 Derek Lewis, Environmental Health & Safety Technician 3-______ Building Supervisor State 24-hour warning point for HAZMAT Spill Notification If you need to report a spill in accordance with SARA Title III Section 304 and KRS 39E.190, please contact the Duty Officer at the Commonwealth Emergency Operations Center at 800.255.2587 which serves as the twenty-four (24) hour warning point and contact for the Commonwealth Emergency Response Commission. Updated 2018 All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboratory Standard 29 CFR 19190.1450. Morehead State UniversityMorehead laboratories State may Chemical use this document Hygiene Plan as a starting point for creating their work area specific Chemical Hygiene Plan (CHP). MSU Laboratory Awareness Certification For CHP of (Professor, Building, Room)________________________ The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Morehead State University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Morehead State University.