1 Titan's Organic Aerosols: Molecular Composition And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”
chemengineering Article Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy” Robert J. Meier Pro-Deo Consultant, 52525 Heinsberg, North-Rhine Westphalia, Germany; [email protected] Abstract: Group contribution (GC) methods to predict thermochemical properties are of eminent importance to process design. Compared to previous works, we present an improved group contri- bution parametrization for the heat of formation of organic molecules exhibiting chemical accuracy, i.e., a maximum 1 kcal/mol (4.2 kJ/mol) difference between the experiment and model, while, at the same time, minimizing the number of parameters. The latter is extremely important as too many parameters lead to overfitting and, therewith, to more or less serious incorrect predictions for molecules that were not within the data set used for parametrization. Moreover, it was found to be important to explicitly account for common chemical knowledge, e.g., geminal effects or ring strain. The group-related parameters were determined step-wise: first, alkanes only, and then only one additional group in the next class of molecules. This ensures unique and optimal parameter values for each chemical group. All data will be made available, enabling other researchers to extend the set to other classes of molecules. Keywords: enthalpy of formation; thermodynamics; molecular modeling; group contribution method; quantum mechanical method; chemical accuracy; process design Citation: Meier, R.J. Group Contribution Revisited: The Enthalpy of Formation of Organic Compounds with “Chemical Accuracy”. 1. Introduction ChemEngineering 2021, 5, 24. To understand chemical reactivity and/or chemical equilibria, knowledge of thermo- o https://doi.org/10.3390/ dynamic properties such as gas-phase standard enthalpy of formation DfH gas is a necessity. -
![United States Patent (19) [11] 3,932,402 Norell (45) Jan](https://docslib.b-cdn.net/cover/6379/united-states-patent-19-11-3-932-402-norell-45-jan-626379.webp)
United States Patent (19) [11] 3,932,402 Norell (45) Jan
United States Patent (19) [11] 3,932,402 Norell (45) Jan. 13, 1976 54 PREPARATION OF SYM-TRAZINES BY 3,071,586 111963 Sandner et al...................... 260/248 TRIMERIZATION OF NITRLES IN 3,095,414 6/1963 Spain hour........................... 260/248 3,238,138 3/1966 Braunwarth et al... ... 260/248 X TRIFLUOROMETHANESULFONIC ACD 3,654, 192 4/1972 Vogel.................................. 260/2 R 75 Inventor: John R. Norell, Bartlesville, Okla. OTHER PUBLICATIONS 73 Assignee: Phillips Petroleum Company, Cool et al., J. Chem. Soc., 1941, pp. 278-282. Bartlesville, Okla. Elderfield, “Heterocyclic Compounds", Vol. 7, Ch. 7, pp. 642, 644 and 686. 22) Filed: June 24, 1974 Migrdichian, "The Chemistry of Organic Cyanogen 21 ) Appl. No.: 482,257 Compounds,' Ch. 17, pp. 356, 357 and 367. (52) U.S. Cl............................................ 260/248 CS Primary Examiner-John M. Ford 51) Int. Cl’........................................ C07D 251/24 58 Field of Search............................... 260/248 CS 57 ABSTRACT Aliphatic and aromatic nitriles are trimerized to sub 56) References Cited stituted symmetrical triazines in the presence of UNITED STATES PATENTS trifluoromethanesulfonic acid. 3,060, 179 lof 1962 Toland................................ 260/248 16 Claims, No Drawings 3,932,402 2 In one embodiment of this invention, the organic PREPARATION OF SYM-TRAZINES BY nitrile is an aliphatic nitrile. The product of this process TRIMERIZATION OF NITRILES IN is a 2,4,6-trialkyl-1,3,5-triazine. TRFLUOROMETHANESULFONIC ACID In another embodiment of this invention, the organic This invention relates to substituted sym-triazines. In 5 nitrile is an aromatic or substituted aromatic nitrile in one aspect it relates to the preparation of alkyl-sub which case the product of the process is a 2,4,6-triaryl stituted sym-triazines. -

Supporting Information
Photocatalytic Atom Transfer Radical Addition to Olefins utilizing Novel Photocatalysts Errika Voutyritsa, Ierasia Triandafillidi, Nikolaos V. Tzouras, Nikolaos F. Nikitas, Eleftherios K. Pefkianakis, Georgios C. Vougioukalakis* and Christoforos G. Kokotos* Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece SUPPORTING INFORMATION Page General Remarks S2 Optimization of the Reaction Conditions for the Photocatalytic Reaction S3 between 1-decene and BrCH2CN Synthesis of Photocatalysts S5 Synthesis of the Starting Materials S12 General Procedure for the Photocatalytic Reaction between Olefins and S18 BrCH2CN General Procedure for the Photocatalytic Reaction between Olefins and S29 BrCCl3 Determination of the Quantum Yield S33 Phosphorescence Quenching Studies S36 References S41 NMR Spectra S43 E. Voutyritsa, I. Triandafillidi, N. V. Tzouras, N. F. Nikitas, E. K. Pefkianakis, G. C. Vougioukalakis & C. G. Kokotos S1 General Remarks Chromatographic purification of products was accomplished using forced-flow ® chromatography on Merck Kieselgel 60 F254 230-400 mesh. Thin-layer chromatography (TLC) was performed on aluminum backed silica plates (0.2 mm, 60 F254). Visualization of the developed chromatogram was performed by fluorescence quenching, using phosphomolybdic acid, anisaldehyde or potassium permanganate stains. Mass spectra (ESI) were recorded on a Finningan® Surveyor MSQ LC-MS spectrometer. HRMS spectra were recorded on Bruker® Maxis Impact QTOF spectrometer. 1H and 13C NMR spectra were recorded on Varian® Mercury (200 MHz and 50 MHz respectively), or a Bruker® Avance (500 MHz and 125 MHz), and are internally referenced to residual solvent signals. Data for 1H NMR are reported as follows: chemical shift (δ ppm), integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br s = broad singlet), coupling constant and assignment. -
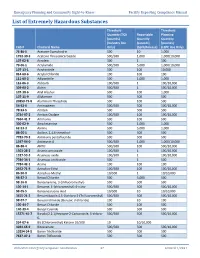
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Natural Product Isolation – How to Get from Biological Material to Pure Compounds Cite This: Nat
NPR View Article Online REVIEW View Journal | View Issue Natural product isolation – how to get from biological material to pure compounds Cite this: Nat. Prod. Rep., 2013, 30, 525 Franz Bucar,*a Abraham Wubea and Martin Schmidb Covering: 2008 to 2012 Since the last comprehensive review by Otto Sticher on natural product isolation in NPR (O. Sticher, Nat. Prod. Rep., 2008, 25, 517), a plethora of new reports on isolation of secondary compounds from higher plants, marine organisms and microorganisms has been published. Although methods described earlier like the liquid-solid chromatographic techniques (VLC, FC, MPLC, HPLC) or partition chromatographic methods are still the major tools for isolating pure compounds, some developments like hydrophilic interaction chromatography (HILIC) have not been fully covered in previous reviews. Furthermore, examples of using different preparative solid-phase extraction (SPE) columns including molecular imprinting technology have been included. Special attention is given to chiral stationary phases in fi Received 25th October 2012 isolation of natural products. Methods for proper identi cation of plant material, problems of post- harvest changes in plant material, extraction methods including application of ionic liquids, de- DOI: 10.1039/c3np20106f replication procedures during natural product isolation are further issues to be discussed by the review. www.rsc.org/npr Selected work published between 2008 and mid-2012 is covered. 1 Introduction 4.2 Column chromatographic methods 2 Authentication and preparation of plant material/ 4.2.1 Vacuum liquid chromatography (VLC) marine organisms 4.2.2 Flash chromatography (FC) 2.1 Morphological/anatomical analysis 4.2.3 Low-pressure liquid chromatography (LPLC) 2.2 TLC/HPTLC analysis 4.2.4 Medium-pressure liquid chromatography (MPLC) Open Access Article. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Laboratory and Chemical Storage Areas Must Be Kept Locked When Not in Use
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ EMERGENCY TELEPHONE NUMBERS: 911 for Emergency and urgent consultation 3-2035 MSU Police; 3-2066 Facilities Management 3-2179 or (606) 207-0629 Eddie Frazier, Director of Risk Management 3-2584 or (606) 207-9425 Holly Niehoff, Environmental Health & Safety Specialist 3-2099 Derek Lewis, Environmental Health & Safety Technician 3-______ Building Supervisor State 24-hour warning point for HAZMAT Spill Notification If you need to report a spill in accordance with SARA Title III Section 304 and KRS 39E.190, please contact the Duty Officer at the Commonwealth Emergency Operations Center at 800.255.2587 which serves as the twenty-four (24) hour warning point and contact for the Commonwealth Emergency Response Commission. Updated 2018 All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboratory Standard 29 CFR 19190.1450. Morehead State UniversityMorehead laboratories State may Chemical use this document Hygiene Plan as a starting point for creating their work area specific Chemical Hygiene Plan (CHP). MSU Laboratory Awareness Certification For CHP of (Professor, Building, Room)________________________ The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Morehead State University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Morehead State University. -

Safety Data Sheet
1/7 Isobutyronitrile,1181E-2,28/07/2020 Date of issue: 05/02/2019 Date of revision: 28/07/2020 Safety Data Sheet 1. Identification of the substance/mixture and of the company/undertaking Product identifier: Product name: Isobutyronitrile SDS No. : 1181E-2 Details of the supplier of the safety data sheet Manufacturer/Supplier: KISHIDA CHEMICAL CO., LTD. Address: 3-1, Honmachibashi, Chuo-ku,Osaka ,JAPAN Division: Safety Management Dept. of Chemicals Telephone number: +81-6-6946-8061 FAX: +81-6-6946-1607 e-mail address: [email protected] 2. Hazards identification GHS classification and label elements of the product Classification of the substance or mixture PHYSICAL AND CHEMICAL HAZARDS Flammable liquids: Category 2 HEALTH HAZARDS Acute toxicity (Oral): Category 3 Acute toxicity (Dermal): Category 2 Acute toxicity (Inhalation): Category 3 Serious eye damage/eye irritation: Category 2 Specific target organ toxicity - single exposure: Category 2(liver) Specific target organ toxicity - single exposure: Category 3 (Respiratory tract irritation) (Note) GHS classification without description: Not classified/Classification not possible Label elements Signal word: Danger HAZARD STATEMENT Highly flammable liquid and vapor Toxic if swallowed Fatal in contact with skin Toxic if inhaled Causes serious eye irritation May cause damage to organs after single exposure(liver) May cause respiratory irritation PRECAUTIONARY STATEMENT Prevention Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep container tightly closed. Ground/bond container and receiving equipment. Use explosion-proof electrical/ventilating/lighting equipment. Use only non-sparking tools. Take precautionary measures against static discharge. Do not breathe dust/fume/gas/mist/vapors/spray. -

Basics of Pump-And-Treat Ground-Water Remediation Technology
United States Robert S. Kerr EPA/600/8-90/003 Environmental Protection Environmental Research Laboratory March 1990 Agency Ada OK 74820 Research and Development Basics of Pump-and-Treat Ground-Water Remediation Technology Word-searchable version – Not a true copy EPA-600/8-90/003 Basics of Pump-and-Treat Ground-Water Remediation Technology James W. Mercer, David C. Skipp and Daniel Giffin Geo Trans, Inc. 250-A Exchange Place Herndon, Virginia 22070 Project Officer Randall R. Ross Extramural Activities and Assistance Division Robert S. Kerr Environmental Research Laboratory Office of Research and Development U.S. Environment Protection Agency Ada, Oklahoma 74820 Word-Searchable Version – Not a true copy Disclaimer The Information in this document has been funded in part by the United States Environmental Protection Agency under Contract No. 68-C8-0058 to Dynamac Corporation. It has been subjected to the Agency's peer and administrative review, and it has been approved for publication as an EPA document. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii Word-searchable version – Not a true copy Foreword EPA is charged by Congress to protect the nation's land, air and water systems. Under a mandate of national environmental laws focused on air and water quality, solid waste management and the control of toxic substances, pesticides, noise and radiation, the Agency strives to formulate and implement actions which lead to a compatible balance between human activities and the ability of natural systems to support and nurture life. The Robert S. Kerr Environmental Research Laboratory is the Agency's center of expertise for investigation of the soil and subsurface environment. -

Environmental Protection Agency Pt. 355, App. B
Environmental Protection Agency Pt. 355, App. B [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 5344–82–1 ............ Thiourea, (2-Chlorophenyl)- ....................................... ..................... 100 100/10,000 614–78–8 .............. Thiourea, (2-Methylphenyl)- ....................................... ..................... 500 500/10,000 7550–45–0 ............ Titanium Tetrachloride ................................................ ..................... 1,000 100 584–84–9 .............. Toluene 2,4-Diisocyanate ........................................... ..................... 100 500 91–08–7 ................ Toluene 2,6-Diisocyanate ........................................... ..................... 100 100 110–57–6 .............. Trans-1,4-Dichlorobutene ........................................... ..................... 500 500 1031–47–6 ............ Triamiphos .................................................................. ..................... 500 500/10,000 24017–47–8 .......... Triazofos ..................................................................... ..................... 500 500 76–02–8 ................ Trichloroacetyl Chloride .............................................. ..................... 500 500 115–21–9 .............. Trichloroethylsilane ..................................................... d .................. 500 500 327–98–0 .............. Trichloronate ............................................................... e ................. -

Chemicals Subject to TSCA Section 12(B) Export Notification Requirements (January 16, 2020)
Chemicals Subject to TSCA Section 12(b) Export Notification Requirements (January 16, 2020) All of the chemical substances appearing on this list are subject to the Toxic Substances Control Act (TSCA) section 12(b) export notification requirements delineated at 40 CFR part 707, subpart D. The chemicals in the following tables are listed under three (3) sections: Substances to be reported by Notification Name; Substances to be reported by Mixture and Notification Name; and Category Tables. TSCA Regulatory Actions Triggering Section 12(b) Export Notification TSCA section 12(b) requires any person who exports or intends to export a chemical substance or mixture to notify the Environmental Protection Agency (EPA) of such exportation if any of the following actions have been taken under TSCA with respect to that chemical substance or mixture: (1) data are required under section 4 or 5(b), (2) an order has been issued under section 5, (3) a rule has been proposed or promulgated under section 5 or 6, or (4) an action is pending, or relief has been granted under section 5 or 7. Other Section 12(b) Export Notification Considerations The following additional provisions are included in the Agency's regulations implementing section 12(b) of TSCA (i.e. 40 CFR part 707, subpart D): (a) No notice of export will be required for articles, except PCB articles, unless the Agency so requires in the context of individual section 5, 6, or 7 actions. (b) Any person who exports or intends to export polychlorinated biphenyls (PCBs) or PCB articles, for any purpose other than disposal, shall notify EPA of such intent or exportation under section 12(b).