1.1 Introduction to Vitamin B 12
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methionine Synthase Supports Tumor Tetrahydrofolate Pools
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Methionine synthase supports tumor tetrahydrofolate pools Joshua Z. Wang1,2,#, Jonathan M. Ghergurovich1,3,#, Lifeng Yang1,2, and Joshua D. Rabinowitz1,2,* 1 Lewis-Sigler Institute for Integrative Genomics, Princeton University, Princeton, New Jersey, USA 2 Department of Chemistry, Princeton University, Princeton, New Jersey, USA 3 Department of Molecular Biology, Princeton University, Princeton, New Jersey, USA # These authors contributed equally to this work. *Corresponding author: Joshua Rabinowitz Department of Chemistry and the Lewis-Sigler Institute for Integrative Genomics, Princeton University, Washington Rd, Princeton, NJ 08544, USA Phone: (609) 258-8985; e-mail: [email protected] Conflict of Interest Disclosure: J.D.R. is a paid advisor and stockholder in Kadmon Pharmaceuticals, L.E.A.F. Pharmaceuticals, and Rafael Pharmaceuticals; a paid consultant of Pfizer; a founder, director, and stockholder of Farber Partners and Serien Therapeutics. JDR and JMG are inventors of patents in the area of folate metabolism held by Princeton University. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.05.284521; this version posted September 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Mammalian cells require activated folates to generate nucleotides for growth and division. The most abundant circulating folate species is 5-methyl tetrahydrofolate (5-methyl- THF), which is used to synthesize methionine from homocysteine via the cobalamin-dependent enzyme methionine synthase (MTR). -

Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights
Taylor University Pillars at Taylor University Student Scholarship: Chemistry Chemistry and Biochemistry Fall 2019 Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights David Joshua Ferguson Follow this and additional works at: https://pillars.taylor.edu/chemistry-student Part of the Analytical Chemistry Commons, Inorganic Chemistry Commons, Organic Chemistry Commons, Other Chemistry Commons, and the Physical Chemistry Commons CHEMISTRY THESIS SIGNIFICANCE AND IMPLICATIONS OF VITAMIN B-12 REACTION SCHEMA- ETH ZURICH VARIANT: MECHANISMS AND INSIGHTS DAVID JOSHUA FERGUSON 2019 2 Table of Contents: Chapter 1 6 Chapter 2 17 Chapter 3 40 Chapter 4 59 Chapter 5 82 Chapter 6 118 Chapter 7 122 Appendix References 3 Chapter 1 A. INTRODUCTION. Vitamin B-12 otherwise known as cyanocobalamin is a compound with synthetic elegance. Considering how it is composed of an aromatic macrocyclic corrin there are key features of this molecule that are observed either in its synthesis of in the biochemical reactions it plays a role in whether they be isomerization reactions or transfer reactions. In this paper the focus for the discussion will be on the history, chemical significance and total synthesis of vitamin B12. Even more so the paper will be concentrated one of the two variants of the vitamin B-12 synthesis, namely the ETH Zurich variant spearheaded by Albert Eschenmoser.Examining the structure as a whole it is observed that a large portion of the vitamin B12 is a corrin structure with a cobalt ion in the center of the macrocyclic part, and that same cobalt ion has cyanide ligands. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

The Vitamin B12 Coenzyme
THE VITAMIN B12 COENZYME D. DoLPHIN, A. W. JoHNSON, R. RoDRIGO and N. SHAW Department of Chemistry, University of Nottingham, U.K. INTRODUCTION In 19•58 Barker and his associatesl-3 recognized a new coenzyme which controlled the conversion of glutamate into ß-methylaspartate by Clostridium tetanomorpkim. The coenzyme was shown4 to be related to !f;-vitamin B12, i.e. contair ing an adenine nucleotide grouping in place of the 5,6-dimethyl benziminawle nucleotide of vitamin B12, although similar coenzymes con taining btnziminazole or 5,6-dimethylbenziminazole were produced by growing C. tetanomorphum in the presence of the a ppropriate base5. Other variations of the nucleotide base have been achieved using Propionibacterium arabinosum in the presence of other purines and benziminazoles6• The pres ence of;:he coenzymes in a wide variety of micro-organisms such as several species of Actinomycetes including Streptomyces olivaceus and S. griseus has been dem( mstrated by the glutamate isomerase assay7 or by isolation. I t appears thü Vitamin B12 and its analogues are always biosynthesized in the form of their coenzymes. Preliminary physical and chemical studies sug gested that in the 5,6-dimethylbenzirninazolyl cobamide coenzyme the cyanide gr )up of vitamin B12, cyanocobalamin, was replaced by an adenine nucleoside':, 5, 8 and the determination9 of the complete structure (I; R = 5'-de·)xyadenosyl) of the coenzyme by X-ray analysis revealed the existence c f an essentially covalent bond between the cobalt atom and the S'.. carbon üom of the additional 5'-deoxyadenosine group. The molecule Me CH 2• CO· NH2 In the vitamin 8 12 coenzyme R =5' - deoxyadenosyl = Me Me 539 D. -

Vitamins Minerals Nutrients
vitamins minerals nutrients Vitamin B12 (Cyanocobalamin) Snapshot Monograph Vitamin B12 Nutrient name(s): (Cyanocobalamin) Vitamin B12 Most Frequent Reported Uses: Cyanocobalamin • Homocysteine regulation Methylcobalamin • Neurological health, including Adenosylcobalamin (Cobamamide) diabetic neuropathy, cognitive Hydroxycobalamin (European) function, vascular dementia, stroke prevention • Anemias, including pernicious and megaloblastic • Sulfite sensitivity Cyanocobalamin Introduction: Vitamin B12 was isolated from liver extract in 1948 and reported to control pernicious anemia. Cobalamin is the generic name of vitamin B12 because it contains the heavy metal cobalt, which gives this water-soluble vitamin its red color. Vitamin B12 is an essential growth factor and plays a role in the metabolism of cells, especially those of the gastrointestinal tract, bone marrow, and nervous tissue. Several different cobalamin compounds exhibit vitamin B12 activity. The most stable form is cyanocobalamin, which contains a cyanide group that is well below toxic levels. To become active in the body, cyanocobalamin must be converted to either methylcobalamin or adenosylcobalamin. Adenosylcobalamin is the primary form of vitamin B12 in the liver. © Copyright 2013, Integrative Health Resources, LLC | www.metaboliccode.com A protein in gastric secretions called intrinsic factor binds to vitamin B12 and facilitates its absorption. Without intrinsic factor, only a small percentage of vitamin B12 is absorbed. Once absorbed, relatively large amounts of vitamin B12 can be stored in the liver. The body actually reabsorbs vitamin B12 in the intestines and returns much of it to the liver, allowing for very little to be excreted from the body. However, when there are problems in the intestines, such as the microflora being imbalanced resulting in gastrointestinal inflammation, then vitamin B12 deficiencies can occur. -
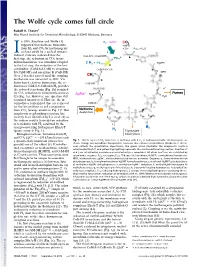
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

The Potential Protective Role of Vitamin K in Diabetic Neuropathy
VITAMINS The potential protective role of vitamin K in diabetic neuropathy DILIP MEHTA Viridis Biopharma 6/10 Jogani Industrial Complex ew cases of diabetes are symptomatic pain relief (3-5). V. N. Purav Marg, Chunabhatti increasing worldwide at a rapid Mumbai 400022, India The etiopathology of peripheral pace, with the total number of neuropathy is poorly understood and many [email protected] people with diabetes was projected factors, including dietary deficiencies, may www.viridisbiopharma.com Nto rise from 171 million in 2000 to 366 million contribute to the clinical manifestation of the in 2030 – an increase of nearly 200 million in condition. Deficiency of vitamin B12 (also only three decades. There are more cases of known as cobalamin), which results in a lack diabetes in women and urban populations, of a related compound, methylcobalamin, is with diabetes in developing countries projected manifested by megaloblastic anemia, and to double in the coming years (1). has been associated with significant Based on reports from the Centers for neurological pathology, especially peripheral Disease Control and Prevention, type 2 neuropathy (6-8). Vitamin B12 is also diabetes dult onset diabetes affects associated with the onset of diabetic approximately 9.3% of the general neuropathy. In patients with diabetic population in the United States in contrast to neuropathy, vitamin B12 deficiency may be 25.9% among those 65 years or older (2). aggravated by the use of antidiabetic agents Diabetes mellitus accounts for 90% of the such as metformin (9-11). Even short-term cases of diabetes patients (3,4). treatment with metformin causes a decrease The prevalence of type 2 diabetes in serum cobalamin, folic acid and an increases with age, higher then 25 body increase in homocysteine, which leads to mass index and the presence of the disease peripheral neuropathy in patients with in family history. -

Potential Benefits of Methylcobalamin: a Review
Open Access Austin Journal of Pharmacology and Therapeutics Review Article Potential Benefits of Methylcobalamin: A Review Gupta JK* and Qureshi Shaiba Sana Department of Pharmacology, GLA University Mathura, Abstract India Methylcobalamin is an active form of vitamin B12 that helps in synthesis *Corresponding author: Jeetendra Kumar Gupta, of methionine and S-adenosylmethionine. It is required for integrity of myelin, Department of Pharmacology, Institute of Pharmaceutical neuronal function, proper red blood cell formation and DNA synthesis. The largest Research, GLA University Mathura, India group of vitamin B12 deficiency is found in typical vegetarians all over the world, which can be alleviated with its analogue Methylcobalamin. It is a beneficial Received: August 17, 2015; Accepted: September 30, drug to most of the common disorders like cardiovascular disorders, diabetes, 2015; Published: October 08, 2015 anemia, hyperhomocysteinemia and degenerative disorders. Methylcobalamin helps in the synthesis of neuronal lipids, regeneration of axonal nerves and has neuroprotective activity, which promote neurons to function in proper way and thus improves Alzheimer disease, Parkinsonism, Dementia and neuropathic syndromes. It is an approved treatment for peripheral neuropathy. Keywords: Mecobalamin; Neuropathy; Anemia; Nootropic; Dietary supplement Abbreviations essential for cell growth and replication. Sometimes the liver cannot convert cyanocobalamin into adequate amount of methylcobalamin SAMe: S-Adenosyl Methionine; ERK: Extracellular Signal- needed for proper neuronal functioning. Through enhanced Regulated Kinases; PKB: Protein Kinase B; B-globulin: Beta Globulin; methylation, it exerts its nerve cell protective effect and accelerates ENFD: Epidermal Nerve Fiber Density; DPN: Diabetic Peripheral its growth. A lot of energy is required for cyanocobalamin to remove Neuropathy; NSAIDs: Non Steroidal Anti Inflammatory Drugs; THF: its cyanide and replaces it with methyl group [3]. -

Vitamin and Mineral Safety 3Rd Edition (2013) Council for Responsible Nutrition (CRN)
EXCERPTED FROM: Vitamin and Mineral Safety 3rd Edition (2013) Council for Responsible Nutrition (CRN) www.crnusa.org Vitamin B12 Introduction Vitamin B12 helps maintain the body’s nervous system and blood cells and supports the production of DNA. Vitamin B12 also helps prevents a type of anemia and has been termed the “anti-pernicious anemia dietary factor.” Vitamin B12 is also the only known physiologically important compound that contains cobalt, and therefore the various forms of vitamin B12 are known collectively as cobalamins. Vitamin B12 is a cofactor in two enzymes that are fundamental in facilitating growth in humans. In the methylcobalamin form, vitamin B12 is the direct cofactor for methionine synthetase, the enzyme that recycles homocysteine back to methionine. Here, vitamin B12 and folic acid have closely related roles in one-carbon metabolism. In the adenosylcobalamin form, vitamin B12 is the cofactor in methylmalonyl-coenzyme A mutase. Both reactions are involved in promoting the rapid growth and proliferation of bone marrow cells and ultimately red blood cells (Expert Group on Vitamins and Minerals [EVM] 2003). Vitamin B12 is essential for the function and maintenance of the central nervous system, and severe deficiency in persons with pernicious anemia produces the neurological disease of posterolateral spinal cord degeneration (Herbert and Das 1994). The direct cause of pernicious anemia, in fact, is vitamin B12 deficiency, but the underlying defect is the absence of an intrinsic factor produced by specific stomach cells and needed for intestinal absorption of vitamin B12. Without this intrinsic factor, absorption is greatly reduced or fails, and a severe and persistent deficiency develops that is not preventable by the usual dietary levels of vitamin B12. -

Magnesium-Protoporphyrin Chelatase of Rhodobacter
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 1941-1944, March 1995 Biochemistry Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: Reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli (protoporphyrin IX/tetrapyrrole/chlorophyll/bacteriochlorophyll/photosynthesis) LUCIEN C. D. GIBSON*, ROBERT D. WILLOWSt, C. GAMINI KANNANGARAt, DITER VON WETTSTEINt, AND C. NEIL HUNTER* *Krebs Institute for Biomolecular Research and Robert Hill Institute for Photosynthesis, Department of Molecular Biology and Biotechnology, University of Sheffield, Sheffield, S10 2TN, United Kingdom; and tCarlsberg Laboratory, Department of Physiology, Gamle Carlsberg Vej 10, DK-2500 Copenhagen Valby, Denmark Contributed by Diter von Wettstein, November 14, 1994 ABSTRACT Magnesium-protoporphyrin chelatase lies at Escherichia coli and demonstrate that the extracts of the E. coli the branch point of the heme and (bacterio)chlorophyll bio- transformants can convert Mg-protoporphyrin IX to Mg- synthetic pathways. In this work, the photosynthetic bacte- protoporphyrin monomethyl ester (20, 21). Apart from posi- rium Rhodobacter sphaeroides has been used as a model system tively identifying bchM as the gene encoding the Mg- for the study of this reaction. The bchH and the bchI and -D protoporphyrin methyltransferase, this work opens up the genes from R. sphaeroides were expressed in Escherichia coli. possibility of extending this approach to other parts of the When cell-free extracts from strains expressing BchH, BchI, pathway. In this paper, we report the expression of the genes and BchD were combined, the mixture was able to catalyze the bchH, -I, and -D from R. sphaeroides in E. coli: extracts from insertion of Mg into protoporphyrin IX in an ATP-dependent these transformants, when combined in vitro, are highly active manner. -

Essential Trace Elements in Human Health: a Physician's View
Margarita G. Skalnaya, Anatoly V. Skalny ESSENTIAL TRACE ELEMENTS IN HUMAN HEALTH: A PHYSICIAN'S VIEW Reviewers: Philippe Collery, M.D., Ph.D. Ivan V. Radysh, M.D., Ph.D., D.Sc. Tomsk Publishing House of Tomsk State University 2018 2 Essential trace elements in human health UDK 612:577.1 LBC 52.57 S66 Skalnaya Margarita G., Skalny Anatoly V. S66 Essential trace elements in human health: a physician's view. – Tomsk : Publishing House of Tomsk State University, 2018. – 224 p. ISBN 978-5-94621-683-8 Disturbances in trace element homeostasis may result in the development of pathologic states and diseases. The most characteristic patterns of a modern human being are deficiency of essential and excess of toxic trace elements. Such a deficiency frequently occurs due to insufficient trace element content in diets or increased requirements of an organism. All these changes of trace element homeostasis form an individual trace element portrait of a person. Consequently, impaired balance of every trace element should be analyzed in the view of other patterns of trace element portrait. Only personalized approach to diagnosis can meet these requirements and result in successful treatment. Effective management and timely diagnosis of trace element deficiency and toxicity may occur only in the case of adequate assessment of trace element status of every individual based on recent data on trace element metabolism. Therefore, the most recent basic data on participation of essential trace elements in physiological processes, metabolism, routes and volumes of entering to the body, relation to various diseases, medical applications with a special focus on iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), selenium (Se), iodine (I), cobalt (Co), chromium, and molybdenum (Mo) are reviewed.