Oral Granular Cell Tumor in a Brazilian Population
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

Rush University Medical Center, May 2005
TABLE OF CONTENTS Case # Title Page 1. Malignant Spitz’s Nevus 1 2. Giant Congenital Nevus 4 3. Methotrexate Nodulosis 7 4. Apthae with Trisomy 8–positive Myelodysplastic Syndrome 10 5. Kwashiorkor 13 6. “Unknown” 16 7. Gangrenous Cellulitis 17 8. Parry-Romberg Syndrome 21 9. Wegener’s Granulomatosis 24 10. Pediatric CTCL 27 11. Hypopigmented Mycosis Fungoides 30 12. Fabry’s Disease 33 13. Cicatricial Alopecia, Unclassified 37 14. Mastocytoma 40 15. Cutaneous Piloleiomyomas 42 16. Granular Cell Tumor 44 17. Disseminated Blastomycoses 46 18. Neonatal Lupus 49 19. Multiple Lipomas 52 20. Acroangiodermatitis of Mali 54 21. Pigmented Basal Cell Carcinoma (BCC) 57 Page 1 Case #1 CHICAGO DERMATOLOGICAL SOCIETY RUSH UNIVERSITY MEDICAL CENTER CHICAGO, ILLINOIS MAY 18, 2005 CASE PRESENTED BY: Michael D. Tharp, M.D. Lady Dy, M.D., and Darrell W. Gonzales, M.D. History: This 2 year-old white female presented with a one year history of an expanding lesion on her left cheek. There was no history of preceding trauma. The review of systems was normal. Initially the lesion was thought to be a pyogenic granuloma and treated with two courses of pulse dye laser. After no response to treatment, a shave biopsy was performed. Because the histopathology was interpreted as an atypical melanocytic proliferation with Spitzoid features, a conservative, but complete excision with margins was performed. The pathology of this excision was interpreted as malignant melanoma measuring 4.0 mm in thickness. A sentinel lymph node biopsy was subsequently performed and demonstrated focal spindle cells within the subcapsular sinus of a left preauricular lymph node. -

A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated Through 445 Nm Diode Laser
healthcare Case Report A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser Maria Vittoria Viani 1,*, Luigi Corcione 1, Chiara Di Blasio 2, Ronell Bologna-Molina 3 , Paolo Vescovi 1 and Marco Meleti 1 1 Department of Medicine and Surgery, University of Parma, 43126 Parma, Italy; [email protected] (L.C.); [email protected] (P.V.); [email protected] (M.M.) 2 Private practice, Centro Medico Di Blasio, 43121 Parma; Italy; [email protected] 3 Faculty of Dentistry, University of the Republic, 14600 Montevideo, Uruguay; [email protected] * Correspondence: [email protected] Received: 10 June 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Oral granular cell tumor (GCT) is a relatively rare, benign lesion that can easily be misdiagnosed. Particularly, the presence of pseudoepitheliomatous hyperplasia might, in some cases, lead to the hypothesis of squamous cell carcinoma. Surgical excision is the treatment of choice. Recurrence has been reported in up to 15% of cases treated with conventional surgery. Here, we reported a case of GCT of the tongue in a young female patient, which was successfully treated through 445 nm diode laser excision. Laser surgery might reduce bleeding and postoperative pain and may be associated with more rapid healing. Particularly, the vaporization effect on remnant tissues could eliminate GCT cells on the surgical bed, thus hypothetically leading to a lower rate of recurrence. In the present case, complete healing occurred in 1 week, and no recurrence was observed after 6 months. Laser surgery also allows the possibility to obtain second intention healing. -
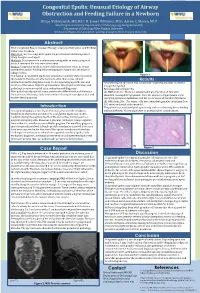
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H. James Williams, MD2; Aaron C. Mason, MD3 1West Virginia University Department of Otolaryngology, Morgantown WV; 2Department of Pathology, West Virginia University 3Division of Plastic, Reconstructive, and Hand Surgery, West Virginia University Abstract Title: Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Objectives: Review congenital epulis; Its presentation and management. Study Design: Case Report Methods: Description of a newborn presenting with an obstructing oral mass. A review of the literature is included. Results: Congenital epulis is a rare oral lesion that may result in airway obstruction and/or feeding failure bringing the mass to the attention of subspecialists. Conclusion: A congenital epulis may present as a solitary alveolar mass in Figure 1 the newborn. Females are affected more often than males. Airway Results obstruction and feeding failure may evolve depending upon the size and The pathological specimen was a maxillary congenital granular cell tumor location of the lesion. Physical examination, radiographic evaluation, and (congenital epulis). pathologic review are useful in its evaluation and diagnosis. Pathology slides (Figure 3): Histopathologically, special stains assist in the differentiation of the lesion (A) H&E stain, 4x: There is a subepithelial proliferation of cells with from other solid tumors. Early intervention relieves airway obstruction and abundant eosinophilic cytoplasm. Note the absence of hyperplasia of the enables feeding success. overlying squamous epithelium and the prominence of vascular structures. (B) H&E stain, 20x: The tumor cells have abundant granular cytoplasm (low N/C ratio) and small uniform nuclei. -

Growing Papule on the Right Shoulder of an Elderly Man
DERMATOPATHOLOGY DIAGNOSIS CLOSE ENCOUNTERS WITH THE ENVIRONMENT Growing Papule on the Right Shoulder of an Elderly Man Campbell L. Stewart, MD; Karolyn A. Wanat, MD; Adam I. Rubin, MD copy A not B H&E, original magnification ×20. H&E, original magnification ×400. Do The best diagnosis is: a. desmoplastic trichilemmoma b. granular cell basal cell carcinoma c. granular cell tumor d. sebaceous adenoma CUTIS e. xanthogranuloma PLEASE TURN TO PAGE 392 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Dr. Stewart is from the University of Washington, Seattle. Dr. Wanat is from the University of Iowa, Iowa City. Dr. Rubin is from the University of Pennsylvania, Philadelphia. The authors report no conflict of interest. Correspondence: Adam I. Rubin, MD, University of Pennsylvania, 2 Maloney Bldg, 3600 Spruce St, Philadelphia, PA 19104 ([email protected]). WWW.CUTIS.COM VOLUME 97, JUNE 2016 391 Copyright Cutis 2016. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Granular Cell Basal Cell Carcinoma asal cell carcinoma (BCC) is the most com- The granules in GBCC generally are positive on peri- mon human epithelial malignancy. There are odic acid–Schiff staining.1-4 several histologic variants, the rarest being The histologic differential diagnosis for GBCC B 1 granular cell BCC (GBCC). Granular cell BCC is includes granular cell tumor as well as other tumors reported most commonly in men with a mean age that can present with granular cell changes such as of 63 years. Sixty-four percent of cases develop on ameloblastoma, leiomyoma, leiomyosarcoma, angio- the face, with the remainder arising on the chest sarcoma, malignant peripheral nerve sheath tumor, or trunk. -

Treatments for Ankyloglossia and Ankyloglossia with Concomitant Lip-Tie Comparative Effectiveness Review Number 149
Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2012-00009-I Prepared by: Vanderbilt Evidence-based Practice Center Nashville, TN Investigators: David O. Francis, M.D., M.S. Sivakumar Chinnadurai, M.D., M.P.H. Anna Morad, M.D. Richard A. Epstein, Ph.D., M.P.H. Sahar Kohanim, M.D. Shanthi Krishnaswami, M.B.B.S., M.P.H. Nila A. Sathe, M.A., M.L.I.S. Melissa L. McPheeters, Ph.D., M.P.H. AHRQ Publication No. 15-EHC011-EF May 2015 This report is based on research conducted by the Vanderbilt Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2012-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

Pigmented Villonodular Synovitis of the Temporomandibular Joint: a Case Report and the Literature Review
1314 Cai et al. Case Report TMJ Disorders J. Cai1, Z. Cai1, Y. Gao2 1Department of Oral and Maxillofacial Pigmented villonodular synovitis 2 Surgery, Beijing, China; Department of Oral Pathology, Peking University School & of the temporomandibular joint: Hospital of Stomatology, Beijing, China a case report and the literature review J. Cai, Z. Cai, Y. Gao: Pigmented villonodular synovitis of the temporomandibular joint: a case report and the literature review. Int. J. Oral Maxillofac. Surg. 2011; 40: 1314–1322. # 2011 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved. Abstract. Pigmented villonodular synovitis (PVNS) is an uncommon benign proliferative disorder of synovium that may involve joints, tendon sheaths, and bursae. It most often affects the knees, and less frequently involves other joints. It presents in the temporomandibular joints (TMJs) extremely rarely. The authors report an elderly female patient with PVNS of the TMJ with skull base extension, who had traumatic history in the same site. It was diagnosed through core-needle Keywords: pigmented villonodular synovitis (PVNS); synovitis; temporomandibular joint biopsy, which was not documented in the literature. Radical excision and follow-up (TMJ). for 7–8 years was recommended because of the reported malignant transformation and high recurrence rate. This case and previously reported cases in the literature are Accepted for publication 2 March 2011 reviewed and discussed. Available online 6 April 2011 The term pigmented villonodular synovi- were reported in detail (Table 1). The visits and mouth-opening for a long time tis (PVNS) was introduced by JAFFE et al. authors present an additional case of during the operation. -
Granular Cell Tumor of the Tongue: a Case Report with Emphasis on The
ancer C C as & e y Ottoman, Oncol Cancer Case Rep 2015,1:1 g R o e l p o o c r t n Oncology and Cancer Case O ISSN: 2471-8556 Reports ResearchCase Report Article OpenOpen Access Access Granular Cell Tumor of the Tongue: A Case Report with Emphasis on the Diagnostic and Therapeutic Proceedings Bacem AE Ottoman* 1Department of Medicine, Division of Hematology/Oncology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA 2Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA Abstract Granular cell tumor (GCT), eponymically Abrikossoff’s myoblastoma, is an uncommon asymptomatic benign neoplasm with controversial etiopathogenia. The tumor typically reveals itself as a well-circumscribed, slowly growing nodular mass. Tongue is most commonly preferred in the head and neck region. The conventional size of the granular cell tumor is usually measuring 2-3 centimeters in its greater diameter. The granular cell tumor can taint all age groups, with a peak between 40 and 60 years. This report introduces, however, a case of GCT of the tongue in a much younger female that was totally excised. The clientele has shown up after 1, 3 and 6 months for follow-up. In the head and neck region, there was no evidence of either recurrence or metachronous clinical manifestations of any similar lesions; at the clinical and sonographical assessment. Keywords: Abrikossoff’s tumor; Granular cell tumor; Myoblastoma; Tongue ultrasound Introduction In 1926, Alexei Ivanovich Abrikossoff has introduced the granular cell tumor (GCT), designating it as a myoblastoma. GCT is an uncommon asymptomatic sessile nodule with typical pink overlying mucosa. -

Description Concept ID Synonyms Definition
Description Concept ID Synonyms Definition Category ABNORMALITIES OF TEETH 426390 Subcategory Cementum Defect 399115 Cementum aplasia 346218 Absence or paucity of cellular cementum (seen in hypophosphatasia) Cementum hypoplasia 180000 Hypocementosis Disturbance in structure of cementum, often seen in Juvenile periodontitis Florid cemento-osseous dysplasia 958771 Familial multiple cementoma; Florid osseous dysplasia Diffuse, multifocal cementosseous dysplasia Hypercementosis (Cementation 901056 Cementation hyperplasia; Cementosis; Cementum An idiopathic, non-neoplastic condition characterized by the excessive hyperplasia) hyperplasia buildup of normal cementum (calcified tissue) on the roots of one or more teeth Hypophosphatasia 976620 Hypophosphatasia mild; Phosphoethanol-aminuria Cementum defect; Autosomal recessive hereditary disease characterized by deficiency of alkaline phosphatase Odontohypophosphatasia 976622 Hypophosphatasia in which dental findings are the predominant manifestations of the disease Pulp sclerosis 179199 Dentin sclerosis Dentinal reaction to aging OR mild irritation Subcategory Dentin Defect 515523 Dentinogenesis imperfecta (Shell Teeth) 856459 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield I 977473 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; Autosomal dominant genetic disorder of tooth development Dentinogenesis Imperfecta - Shield II 976722 Dentin, Hereditary Opalescent; Shell Teeth Dentin Defect; -

1 Pulmonary Pathology Journal Club
Pulmonary Pathology Journal Club November 25, 2019 (Joanne) Eunhee S. Yi, MD and Matt Cecchini, MD, PhD Mayo Clinic, Rochester, MN Table of Contents Articles for Discussion Page 4 Larsen BT et al. Clinical and histopathologic features of immune check point inhibitor-related pneumonitis. Am J Surg Pathol 2019;43:1331-40 Page 5 Offin M et al. Current RB and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol 2019;14: 1784-93 Page 6 Roschel FM et al. Feasibility of perioperative-computed tomography of human lung cancer specimens. A pilot study. Arch Pathol Lab Med 2019;143:319-25 Page 7 Zhang et al. Clinical significance of the cribriform pattern in invasive adenocarcinoma of the lung. J Clin Pathol 2019;72:682-8 Page 8 Chae KW et al. Clinical and immunological implications of frameshift mutations in lung cancer. J Thorac Oncol 2019;14:1807-17 Articles for Notation Neoplastic Page 9 Choe EA et al. Dynamic changes in PD-L1 expression and CD8+ T cell infiltration in non-small cell lung cancer following chemoradiation therapy. Lung Cancer 2019;136:30-6 Page 10 Jin J et al. Diminishing microbiome richness and distinction in the lower respiratory tract of lung cancer patients: A multiple comparative study design with independent validation. Lung Cancer 2019;136:129-35 Page 10 Davis R et al. Pulmonary granular cell tumors. A study of 4 cases including a malignant phenotype. Am J Surg Pathol 2019;43:1397-1402 Page 11 Song Z et al.