Copyrighted Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5.111 Principles of Chemical Science, Fall 2005 Transcript – Lecture 28
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Please use the following citation format: Sylvia Ceyer and Catherine Drennan, 5.111 Principles of Chemical Science, Fall 2005. (Massachusetts Institute of Technology: MIT OpenCourseWare). http://ocw.mit.edu (accessed MM DD, YYYY). License: Creative Commons Attribution-Noncommercial-Share Alike. Note: Please use the actual date you accessed this material in your citation. For more information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science, Fall 2005 Transcript – Lecture 28 All right. We have a few more PowerPoint things before I am going to attempt using the board. Again, we are in the transition metal unit. And today we are going to introduce something called crystal field theory. And this is in Chapter 16 in your book. Let me just tell you about two different theories. Again, chemistry is an experimental science. We collect data and then we try to come up with theories that explain the data, so the theories are not sort of 100%. And some are more simple and some are more complicated to try to explain what we observe. And some of these theories, although they are pretty simple approximations of what is really going on, do a pretty good job of explaining the things that we are observing. All right. There are two different kinds of theories that you will hear about with transition metals. You will hear about crystal field theory and ligand field theory. -

FAROOK COLLEGE (Autonomous)
FAROOK COLLEGE (Autonomous) M.Sc. DEGREE PROGRAMME IN CHEMISTRY CHOICE BASED CREDIT AND SEMESTER SYSTEM-PG (FCCBCSSPG-2019) SCHEME AND SYLLABI 2019 ADMISSION ONWARDS 1 CERTIFICATE I hereby certify that the documents attached are the bona fide copies of the syllabus of M.Sc. Chemistry Programme to be effective from the academic year 2019-20 onwards. Date: Place: P R I N C I P A L 2 FAROOK COLLEGE (AUTONOMOUS) MSc. CHEMISTRY (CSS PATTERN) Regulations and Syllabus with effect from 2019 admission Pattern of the Programme a. The name of the programme shall be M.Sc. Chemistry under CSS pattern. b. The programme shall be offered in four semesters within a period of two academic years. c. Eligibility for admission will be as per the rules laid down by the College from time to time. d. Details of the courses offered for the programme are given in Table 1. The programme shall be conducted in accordance with the programme pattern, scheme of examination and syllabus prescribed. Of the 25 hours per week, 13 hours shall be allotted for theory and 12 hours for practical; 1 theory hour per week during even semesters shall be allotted for seminar. Theory Courses In the first three semesters, there will be four theory courses; and in the fourth semester, three theory courses. All the theory courses in the first and second semesters are core courses. In the third semester there will be three core theory courses and one elective theory course. College can choose any one of the elective courses given in Table 1. -

Organometallic Compounds
Chapter 11 Organometallic compounds Organometallics Reactions using organometallics Organometallic compounds Ch 11 #2 = comp’s containing a carbon-metal bond δ− δ+ C in organometallic comp’ds are nucleophilic. C in organic comp’d (like ROH, RNH , and RX) 2 δ+ δ are electrophilic. − due to ∆EN Ch 11 #3 in substitution reactions a carbon Nu: R-Li and R-MgX Ch 11 #4 (used to be) the two most common organometallics organolithium comp’ds BuLi = an alkyl lithium organomagnesium comp’ds = Grignard reagents 1912 Nobel Prize R, Ar, vinyl all possible; Br (as X) popular Ether (solvent) coordinates Mg, stabilizing it. Reactions of R-Li and R-MgX Ch 11 #5 reacts like a carbanion C:– ~ a C Nu: reactions as C Nu: (like SN(2)) nucleophilic addition to carbonyls ~ more often Chapt 16 Ch 11 #6 R-Li and R-MgX are very strong B: how strong? pKa? − − react even with very weak acid stronger than OH? NH2? useful for preparing deuterated HC Storage and reaction must be acid- and moisture-free. Transmetal(l)ation Ch 11 #7 R-Li is more reactive than R-MgX is. C–Li more polar than C–Mg C of R-Li more nu-philic [better Nu:] transmetalation [metal exchange] to less reactive [more stable] organometallic Coupling using Gilman reagent Ch 11 #8 coupling reaction (in organic chemistry) two hydrocarbon fragments are coupled (to form C–C) with the aid of a (transition) metal catalyst Gilman reagent coupling of R of R-X and R’ of Gilman reagent RX + R’2CuLi R–R’ mechanism? substitution of X with R’? not clear Ch 11 #9 R can be alkyl, aryl, or alkenyl [vinyl] which is not possible by R-Li or R-MgX Is R of Gilman why? they are SN(2). -
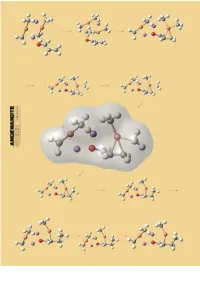
Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry
REVIEWS Wherefore Art Thou Copper? Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry Eiichi Nakamura* and Seiji Mori Organocopper reagents provide the principles. This review will summarize example of molecular recognition and most general synthetic tools in organic first the general structural features of supramolecular chemistry, which chemistry for nucleophilic delivery of organocopper compounds and the pre- chemists have long exploited without hard carbanions to electrophilic car- vious mechanistic arguments, and then knowing it. Reasoning about the bon centers. A number of structural describe the most recent mechanistic uniqueness of the copper atom among and mechanistic studies have been pictures obtained through high-level neighboring metal elements in the reported and have led to a wide variety quantum mechanical calculations for periodic table will be presented. of mechanistic proposals, some of three typical organocuprate reactions, which might even be contradictory to carbocupration, conjugate addition, Keywords: catalysis ´ conjugate addi- others. With the recent advent of and SN2 alkylation. The unified view tions ´ copper ´ density functional physical and theoretical methodolo- on the nucleophilic reactivities of met- calculations ´ supramolecular chemis- gies, the accumulated knowledge on al organocuprate clusters thus ob- try organocopper chemistry is being put tained has indicated that organocup- together into a few major mechanistic rate chemistry represents an intricate 1. Introduction 1 R Cu X The desire to learn about the nature of elements has been R or R1 R and will remain a main concern of chemists. In this review, we R Cu will consider what properties of copper make organocopper R1 chemistry so useful in organic chemistry. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -

Organometallic and Catalysis
ORGANOMETALLIC AND CATALYSIS Dr. Malay Dolai, Assistant Professor, Department of Chemistry, Prabhat Kumar College, Contai, Purba Medinipur-721404, WB, India. 1.Introduction Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and tin, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. In 1827, Zeise's salt is the first platinum- olefin complex: K[PtCl3(C2H4)].H2O, the first invented organometallic compound. Organometallic compounds find wide use in commercial reactions, both as homogeneous catalysis and as stoichiometric reagents For instance, organolithium, organomagnesium, and organoaluminium compounds, examples of which are highly basic and highly reducing, are useful stoichiometrically, but also catalyze many polymerization reactions. Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as carbonylations. The production of acetic acid from methanol and carbon monoxide is catalyzed via metal carbonyl complexes in the Monsanto process and Cativa process. Most synthetic aldehydes are produced via hydroformylation. The bulk of the synthetic alcohols, at least those larger than ethanol, are produced by hydrogenation of hydroformylation- derived aldehydes. -

Mónia Andreia Rodrigues Martins Estudos Para O
Universidade de Aveiro Departamento de Química 2017 MÓNIA ANDREIA ESTUDOS PARA O DESENVOLVIMENTO DE NOVOS RODRIGUES MARTINS PROCESSOS DE SEPARAÇÃO COM TERPENOS E SUA DISTRIBUIÇÃO AMBIENTAL STUDIES FOR THE DEVELOPMENT OF NEW SEPARATION PROCESSES WITH TERPENES AND THEIR ENVIRONMENTAL DISTRIBUTION Universidade de Aveiro Departamento de Química 2017 MÓNIA ANDREIA ESTUDOS PARA O DESENVOLVIMENTO DE NOVOS RODRIGUES MARTINS PROCESSOS DE SEPARAÇÃO COM TERPENOS E SUA DISTRIBUIÇÃO AMBIENTAL STUDIES FOR THE DEVELOPMENT OF NEW SEPARATION PROCESSES WITH TERPENES AND THEIR ENVIRONMENTAL DISTRIBUTION Tese apresentada à Universidade de Aveiro para cumprimento dos requisitos necessários à obtenção do grau de Doutor em Engenharia Química, realizada sob a orientação científica do Professor Doutor João Manuel da Costa e Araújo Pereira Coutinho, Professor Catedrático do Departamento de Química da Universidade de Aveiro, e do Professor Doutor Simão Pedro de Almeida Pinho, Professor Coordenador da Escola Superior de Tecnologia e Gestão do Instituto Politécnico de Bragança. Apoio financeiro da FCT e do FSE no âmbito do III Quadro Comunitário de Apoio (SFRH/BD/87084/2012). Aos meus pais. o júri presidente Prof. Doutor Joaquim Manuel Vieira professor catedrático no Departamento de Engenharia Cerâmica e do Vidro da Universidade de Aveiro Prof. Doutora Isabel Maria Almeida Fonseca professora associada com agregação da Faculdade de Ciências e Tecnologia da Universidade de Coimbra Prof. Doutor Héctor Rodríguez Martínez professor associado do Departamento de Engenharia Química da Universidade de Santiago de Compostela Prof. Doutor Luís Manuel das Neves Belchior Faia dos Santos professor associado da Faculdade de Ciências da Universidade do Porto Prof. Doutor Simão Pedro de Almeida Pinho professor coordenador na Escola Superior de Tecnologia e Gestão do Instituto Politécnico de Bragança Prof. -

Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2
Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2 RED Metal-ligand and metal-metal bonding of the transition metal elements Synopsis Lecture 1: Trends of the transition metal series. Ionic vs Covalent bonding. Nomenclature. Electron counting. Lecture 2: Thermodynamics of complex formation. Why complexes form. Recap of molecular orbital theory. 18-electron rule. Lecture 3: Ligand classes. -donor complexes. Octahedral ML6 molecular orbital energy diagram. Lecture 3: - acceptor ligands and synergic bonding. Binding of CO, CN , N2, O2 and NO. Lecture 4: Alkenes, M(H2) vs M(H)2, Mn(O2) complexes, PR3. Lecture 5: 2- - 2- 3- donor ligands, metal-ligand multiple bonds, O , R2N , RN , N . Lecture 6: ML6 molecular orbital energy diagrams incorporating acceptor and donor ligands. Electron counting revisited and link to spectrochemical series. Lecture 7: Kinetics of complex formation. Substitution mechanisms of inorganic complexes. Isomerisation. Lecture 8: Ligand effects on substitution rates (trans-effect, trans-influence). Metal and geometry effects on substitution rates. Lecture 9: Outer sphere electron transfer. Lecture 10: Inner Sphere electron transfer. Bridging ligands. 2 Learning Objectives: by the end of the course you should be able to i) Use common nomenclature in transition metal chemistry. ii) Count valence electrons and determine metal oxidation state in transition metal complexes. iii) Understand the physical basis of the 18-electron rule. iv) Appreciate the synergic nature of bonding in metal carbonyl complexes. v) Understand the relationship between CO, the 'classic' -acceptor and related ligands such as NO, CN, N2, and alkenes. 2 vi) Describe the nature of the interaction between -bound diatomic molecules (H2, O2) and their relationship to -acceptor ligands. -
![Terpenoids [Compatibility Mode].Pdf](https://docslib.b-cdn.net/cover/8897/terpenoids-compatibility-mode-pdf-1778897.webp)
Terpenoids [Compatibility Mode].Pdf
Terpenoids Dr. Amol Kharat Objectives • To study the Terpenoids in the form of Meaning, Different types Properties, occurrences, uses Isolation Method General Biogenetic Pathway Pharmacognostic account of different drug con taining important constituents Terpenoids. INTRODUCTION Terpenoids are the secondary metabolites synthesized by plants, marine organisms and fungi by head to tail joining of isoprene units . They are also found to occur in rocks, fossils and animal kingdom. Isoprene The terpenoids , sometimes referred to as isoprenoids , are a large and diverse class of naturally-occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in functional groups but also in their basic carbon skeletons . These lipids can be found in all classes of living things, and are the largest group of natural products. CALASSFICATION TYPE OF NUMBER OF ISOPRENE TERPENOIDS CARBON ATOMS UNITS hemiterpene C5 one monoterpenoid C10 two sesquiterpenoid C15 three diterpenoid C20 four sesterterpenoid C25 five triterpenoid C30 six tetraterpenoid C40 eight NOTE hemi = half di = two : sesqui = one and a half tri = three tetra = four Mnonoterpenoids Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C 10 H16 . Monoterpenes may be linear (acyclic) or contain rings. Biochemical modifications such as oxidation or rearrange- ment produce the related monoterpenoids. Mnonoterpenoids Acyclic monoterpenoid: Bicyclic monoterpenoid CHO CH 2 OH n e ro l g era n ia l ¦Â-m y r c e n e ¦Á ---ppp i nene Monocyclic monoterpenodi OH O OH O d-borneol l-menthol menthone cin eole Acyclic monoterpenoid Acyclic monoterpenoid Biosynthetically, isopentenyl pyrophosphate( 异戊烯 焦磷酸) and dimethylallyl pyrophosphate ( 二甲基丙烯焦 磷酸酯) are combined to form geranyl pyrophosphate (牻牛儿醇焦磷酸酯). -

Materials Sciences Programs Fiscal Year 1994
DOE/ER-0648 Dist. Category UC-404 April 1995 Materials Sciences Programs Fiscal Year 1994 U.S. Department of Energy Office ofEnergy Research Office of Basic Energy Sciences Division of Materials Sciences Germantown Building 19901 Germantown Road Germantown, MD 20874-1290 FOREWORD The Division of Materials Sciences is located within the Department of Energy (DOE) in the Office of Basic Energy Sciences which is under the Office of Energy Research. The Director of the Office of Energy Research is appointed by the President and confirmed by the Senate. The Director of the Office of Energy Research is responsible for oversight of, and providing advice to, the Secretary of Energy on the Department's research portfolio and on the management of all of the Laboratories that It owns, except for those that are designated as having a primary role in nuclear weaponry. The Division of Materials Sciences Is responsible for basic research and research facilities in strategic materials science topics of critical importance to the mission of the Department and its Strategic Plan. Other programmatic divisions under the Office of Basic Energy Sciences are Chemical Sciences, Engineering and Geosciences, and Energy Biosciences; information for them is contained on page 165. Materials Science is an enabling technology. The performance parameters, economics, environmental acceptability and safety of all energy generation, conversion, transmission and conservation technologies are limited by the properties and behavior of materials. The Materials Sciences programs develop scientific understanding of the synergistic relationship amongst the synthesis, processing, structure, properties, behavior, performance and other characteristics of materials. Emphasis is placed on the development of the capability to discover technologically, economically, and environmentally desirable new materials and processes, and the instruments and national user facilities necessary for achieving such progress. -

Metal–Dithiolene Bonding Contributions to Pyranopterin Molybdenum Enzyme Reactivity
inorganics Review Metal–Dithiolene Bonding Contributions to Pyranopterin Molybdenum Enzyme Reactivity Jing Yang 1 , John H. Enemark 2 and Martin L. Kirk 1,* 1 Department of Chemistry and Chemical Biology, The University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA; [email protected] 2 Department of Chemistry Biochemistry, University of Arizona, Tucson, AZ 85721, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-505-277-5992 Received: 2 February 2020; Accepted: 2 March 2020; Published: 5 March 2020 Abstract: Here we highlight past work on metal–dithiolene interactions and how the unique electronic structure of the metal–dithiolene unit contributes to both the oxidative and reductive half reactions in pyranopterin molybdenum and tungsten enzymes. The metallodithiolene electronic structures detailed here were interrogated using multiple ground and excited state spectroscopic probes on the enzymes and their small molecule analogs. The spectroscopic results have been interpreted in the context of bonding and spectroscopic calculations, and the pseudo-Jahn–Teller effect. The dithiolene is a unique ligand with respect to its redox active nature, electronic synergy with the pyranopterin component of the molybdenum cofactor, and the ability to undergo chelate ring distortions that control covalency, reduction potential, and reactivity in pyranopterin molybdenum and tungsten enzymes. Keywords: metal–dithiolene; pyranopterin molybdenum enzymes; fold-angle; tungsten enzymes; electronic structure; pseudo-Jahn–Teller effect; thione; molybdenum cofactor; Moco 1. Introduction It is now well-established that all known molybdenum-containing enzymes [1–3], with the sole exception of nitrogenase, contain a common pyranopterin dithiolene (PDT) (Figure1) organic cofactor (originally called molybdopterin (MPT)), which coordinates to the Mo center of the enzymes through the sulfur atoms of the dithiolene fragment. -

Organocuprate Aggregation and Reactivity: Decoding the 'Black Box'
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Organocuprate Aggregation and Reactivity: Decoding the ‘Black Box’ Aliaksei Putau aus Vitebsk, Weißrussland 2012 Erklärung Diese Dissertation wurde im Sinne von § 7 der Promotionsordnung vom 28. November 2011 von Herrn Prof. Dr. Konrad Koszinowski betreut, und von Herrn Prof. Dr. Mayr von der Fakultät für Chemie und Pharmazie vertreten. Eidesstattliche Versicherung Diese Dissertation wurde selbständig, ohne unerlaubte Hilfe erarbeitet. München, 11.06.2012 _______________________ Aliaksei Putau Dissertation eingereicht am: 12.06.2012 1. Gutachter Prof. Dr. Herbert Mayr 2. Gutachter Prof. Dr. Konrad Koszinowski Mündliche Prüfung am: 20.07.2012 Acknowledgements It is a pleasure to thank the many people who made this project possible. My gratitude to my PhD supervisor, Prof. Dr. Konrad Koszinowski, is difficult to overstate. His sound advice, good company, and enthusiasm for trying new things ensured the tropical sunny climate in our group that is so necessary for fruitful (and, above all, enjoyable) research. Besides, his involvement in the group life outside the lab made all of us group members feel like team members, not just a loose bunch of colleagues. I am also deeply indebted to Prof. Dr. Herbert Mayr, not only for his continuous generous support and help, but also for running such a cool, all-star research group, which provided a great many social occasions. I thank SFB 749 for the financial support of this project, specifically Mrs. Birgit Carell, for her support and understanding of my worries and troubles of all kinds. Furthermore, my sincere gratitude goes to Prof.