Differences in Exocuticle Thickness in Leucorrhinia Dubia (Odonata) Larvae from Habitats with and Without Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On Fenn's and Whixall Moss, Shropshire, UK
Recapture rates and habitat associations of White-faced Darter Leucorhinnia dubia on Fenn's and Whixall Moss, Shropshire, UK Item Type Article Authors Davies, Rachel; von Hardenberg, Achaz; Geary, Matthew Citation Recapture rates and habitat associations of White-faced Darter Leucorhinnia dubia on Fenn's and Whixall Moss, Shropshire, UK R Davies, A von Hardenberg, M Geary Journal of the British Dragonfly Society 34 (2), 89-101 Publisher British Dragonfly Society Journal Journal of the British Dragonfly Society Rights Attribution 3.0 United States Download date 28/09/2021 18:54:03 Item License https://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/621511 Recapture rates and habitat associations of Leucorrhinia dubia (White-faced Darter; Vander Linden) on Fenn’s and Whixall Moss, Shropshire, UK Rachel Davies, Achaz von Hardenberg & Matthew Geary* Conservation Biology Research Group, Department of Biological Science, University of Chester, Chester, CH1 4BJ *Corresponding author: [email protected] Abstract Land-use change and habitat loss are important drivers of biodiversity decline at both global and local scales. To protect species from the impacts of land-use change it is important to understand the population dynamics and habitat associations across these scales. Here we present an investigation into the survival and habitat preferences of Leucorrhinia dubia at the local scale at Fenn’s and Whixall Moss, Shropshire, UK. We used mark-release-recapture methods to investigate survival and used sightings of individual dragonflies along with habitat data to investigate habitat preference. We found that survival between capture- visits was very low and that L. -

White Faced Darter © Barry Henwood
White faced darter © Barry Henwood White faced darter Leucorrhinia dubia Vander Linden Climate Change Sensitivity: HIGH Ability to Manage: MEDIUM Non climatic threats: MEDIUM Vulnerability: HIGH Summary The white faced darter has been lost from many sites in the south and midlands. Across its range, the loss and degradation of lowland peat bogs has been the main cause of its decline, but the loss from its southern sites suggests that the warming climate may be having an impact. The species has been well studied, and successfully reintroduced to sites where habitats have been restored. The restoration of former sites or the creation of new sites in parts of the country that will remain climatically suitable for the species will offer the best approach to building the resilience of the species within England. Climate Change Adaptation Manual Evidence to support nature conservation in a changing climate 467 Description The white-faced darter is a small dragonfly with a characteristic white face that gives it its name. The male is mainly black with scarlet and orange markings. Females are also predominantly black but have pale yellow markings. Its dark colouration means that it is able to warm up quickly in the sun, and therefore is well adapted to cool conditions. Ecology and Distribution The white faced darter is a rare dragonfly found mainly in Scotland, but also in the north of England, it has now been lost from all of its southern sites (Pam Taylor pers. comm.). It is a species of lowland peatbogs, and prefers relatively deep, low nutrient, acidic pools with marginal rafts of sphagnum in which to breed. -

Index to Contents
Index to Contents Author(s) Title Year Vol Pages Holland, Sonia Dragonfly Survey Reports – 1. Gloucestershire 1983 1 (1) 1-3 Butler, Stephen Notes on finding larvae of Somatochlora arctica (Zetterstedt) in N. W. Scotland 1983 1 (1) 4-5 Winsland, David Some observations on Erythromma najas (Hansemann) 1983 1 (1) 6 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Vick, G. S. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Merritt, R. Coenagrion mercuriale (Charpentier) with notes on habitat 1983 1 (1) 9-12 Chelmick, D. G. Observations on the ecology and distribution of Oxygastra curtisii (Dale) 1983 1 (2) 11-14 Khan, R. J. Observations of Wood-mice (Apodemus sylvaticus) and Hobby (Falco subbuteo) feeding on dragonflies 1983 1 (2) 15 Marren, P. R. Scarce Species Status Report 2. A review of Coenagrion hastulatum (Charpentier) in Britain 1983 1 (2) 16-19 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (2) 16-19 Mayo, M. C. A. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and River Test in Hampshire 1983 1 (2) 20-21 Welstead, A. R. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and river Test in Hampshire 1983 1 (2) 20-21 Kemp, R. G. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Vick, G. S. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Corbet, P. -

Ebony Boghaunter Williamsonia Fletcheri
Natural Heritage Ebony Boghaunter & Endangered Species Williamsonia fletcheri Program State Status: Endangered www.mass.gov/nhesp Federal Status: None Massachusetts Division of Fisheries & Wildlife DESCRIPTION OF ADULT: The Ebony Boghaunter (Williamsonia fletcheri) is a small, delicately built, blackish dragonfly (order Odonata, sub-order Anisoptera). It is one of the smallest members of the emerald family (Corduliidae). Ebony Boghaunters are dull black in color, with bright green (male) or grey (female) eyes, a metallic brassy green frons (the prominent bulge on the front of the head), and a yellow- brown labium. The black abdomen has a pale yellow- white ring between the 2nd and 3rd abdominal segments (all Odonates have 10 abdominal segments), and a less conspicuous ring between the 3rd and 4th segments. Females are similar to the males, but have thicker abdomens, shorter terminal appendages at the tip of the abdomen, and are paler in coloration. Photo by Michael W. Nelson, NHESP Ebony Boghaunters range in length from 1.2 to 1.5 inches (32 to 34mm), with males averaging slightly The Petite Emerald (Dorocordulia lepida) has a flight larger. The wings are about 1.1 inches (22 mm) long and season overlapping that of Ebony Boghaunter and is hyaline (transparent and colorless), except for a very similar in its dark overall coloration, slight build, and small amber patch at the base. The nymph was metallic green frons with a yellow-brown labium. undescribed until recently (Charlton and Cannings, However, the Petite Emerald is slightly larger with a 1993). When fully developed, the nymphs average about metallic luster on its thorax, lacks abdominal rings, and 0.6 inch (15 to 17mm) in length, and can be identified has brilliant green eyes when mature. -

Panama, by Nick Donnelly
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 23 14 October 2011 Number 3 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 23, No. 3, 14 October 2011 In This Issue .................................................................................................................................................................1 DSA is on Facebook ....................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 2011 Annual Meeting of DSA held in Fort Collins, Colorado, by Dave Leatherman ...............................................2 Northeast Regional DSA Meeting, by Joshua Rose ...................................................................................................8 2011 Annual Oregon Aeshna Blitz Sets New Records, by Steve Gordon .................................................................10 2012 Annual DSA Meeting: Baldcypress Swamps, Sandy Ponds, Blackwater Rivers, and Clubtails, by Chris Hill ....................................................................................................................................................................12 Northeast Meetings Update, by Bryan Pfeiffer .........................................................................................................12 -

Odonata) in Cities Across Central Europe
Eur. J. Entomol. 109: 235–245, 2012 http://www.eje.cz/scripts/viewabstract.php?abstract=1702 ISSN 1210-5759 (print), 1802-8829 (online) Patterns in the diversity of dragonflies (Odonata) in cities across Central Europe CHRISTOPH WILLIGALLA and THOMAS FARTMANN* Department of Community Ecology, Institute of Landscape Ecology, University of Münster, Robert-Koch-Str. 28, 48149 Münster, Germany Key words. Odonata, climate change, environmental gradient, species richness, temperature, urbanisation Abstract. Urbanisation is an important cause of species extinctions. Although urban water systems are also highly modified, studies on aquatic or semi-aquatic organisms are rare. The aim of this study is to identify the factors that determine species richness of Odo- nata in 22 Central European cities and along an urban-rural gradient within six of them. With 64 indigenous species in total and an average of 33 species per city, the species richness of Odonata in Central European cities is comparatively high. A generalised linear model indicates that species richness is positively related to city area. Additional predictors are climatic variables (temperature amplitude, sunshine duration and July temperature) and the year last studied. Since most cities are usually located in areas with natu- rally high habitat heterogeneity, we assume that cities should be naturally rich in dragonflies. The role of city area as a surrogate for habitat and structural richness most likely explains why it is strongly associated with Odonata species richness. The relationship between species richness and the climatic variables probably reflects that Odonata species richness in Central Europe is limited by warm and sunny conditions more than by availability of water. -

Damselflies & Dragonflies of the Cairngorms
Damselflies & Dragonflies of the Cairngorms Male and female Northern damselfly in tandem An identification guide Dragonflies are amazing insects that combine stunning colours with awesome aerial displays. This guide will help you identify the 13 species of dragonfly and damselfly found in and around the Cairngorms National Park. You can get involved and record all these species and help put together a national atlas of dragonflies. Published by The Cairngorms LBAP 1M 2M 3M 1F 2F 3F Damselflies Delicate 2. Large Red Damselfly vegetation usually below the insects, weak fluttery flight. Tot: 33 – 36mm tops of plants, and avoid large Eyes on each side of oblong Range: Cairngorms-wide areas of open water. The head, wings usually held Rarity: common underside of the eyes and closed at rest. Habitat: still or slow face are bright green. Males 1. Emerald Damselfly moving water have two short black lines on Total body length (Tot): Seen: May to August each side of the thorax, and 35 – 39mm A distinctive red and black a spear-shaped mark and 2 Range: Cairngorms-wide damselfly that is often the black lines on the second Rarity: common first to be seen in late spring. abdominal segment. Females Habitat: well vegetated Females are darker with are pea-green with the 2 standing water, ditches and black and yellow bands on thorax lines. loch edges the abdomen. Males defend 4. Common Blue Seen: June to September their territories vigorously. Damselfly A slender species, with a 3. Northern Damselfly Tot: 29 – 36mm weak, fluttery flight. Males Tot: 31 – 33mm Range: Cairngorms-wide are metallic green with blue Range: limited to only 30 Rarity: common segments 9 and 10 on the sites in the Cairngorms Habitat: most wetlands rear of the abdomen. -

1 Common Dragonflies and Damselflies of the Chicago Region
WEB V ERSION Odonata of Northeastern Illinois, USA 1 Common Dragonflies and Damselflies of the Chicago Region Volunteer Stewardship Network – Chicago Wilderness Produced by: John & Jane Balaban, Jennie Kluse & Robin Foster, with assistance of Laurel Ross and support from the Gordon & Betty Moore Foundation. Photos © John & Jane Balaban; [[email protected]] North Branch Restoration Project, with additions by © Thomas Murray (27, 32) and © Vincent Hickey (30). © Environmental & Conservation Programs, The Field Museum, Chicago, IL 60605 USA. [http://www.fmnh.org/chicagoguides/]. Chicago Wilderness Guide #1 version 2 (4/2006) RESOURCES: LIBELLULIDAE - Skimmers Drangonflies of Indiana by J. R. Curry. Large, showy, frequently seen Indiana Academy of Science. 2001. ISBN: 1-883362-11-3 resting on or flying low over Beginner’s Guide to Dragonflies by Nikula and Sones vegetation. Often hunt from a perch with D. and L. Stokes. Little, Brown, and Company. 2002. ISBN: 0-316-81679-5 like Kingbirds. Also includes our Damselflies of the Northeast by E. Lam. Biodiversity smallest dragonflies (Nannothemis Books. 2004. ISBN: 0-9754015-0-5 Damselflies of the North Woods by B. DuBois. and Perithemis) and the ubiquitous Kollath-Stensaas Pub. 2005. ISBN: 0-9673793-7-7 Meadowhawks. http://bugguide.ent.iastate.edu/node/view/191/bgimage 1 Sympetrum rubicundulum / http://cirrusimage.com/dragonflies.htm Ruby Meadowhawk: male and female mating in http://wisconsinbutterflies.org/damselflies/ “wheel” position. 34-38mm 2 Sympetrum obtrusum 3 Sympetrum vicinum 4 Sympetrum semicinctum White-faced Meadowhawk: white face. 32-36mm Yellow-legged Meadowhawk: yellow legs. 30-36mm Band-winged Meadowhawk: half amber wings. 26-38mm Above species are medium-sized and common. -
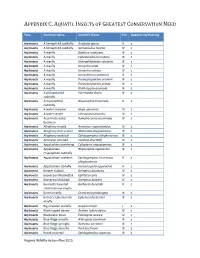
Appendix C. Aquatic Insects of Greatest Conservation Need
APPENDIX C. AQUATIC INSECTS OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Aq Insects A limnephilid caddisfly Anabolia apora II c Aq Insects A limnephilid caddisfly Nemotaulius hostilis IV c Aq Insects A mayfly Baetisca rubescens III c Aq Insects A mayfly Ephemerella inconstans III c Aq Insects A mayfly Habrophlebiodes celeteria III c Aq Insects A mayfly Isonychia arida IV c Aq Insects A mayfly Isonychia serrata IV c Aq Insects A mayfly Isonychia tusculanensis II c Aq Insects A mayfly Paraleptophlebia assimilis III c Aq Insects A mayfly Paraleptophlebia jeanae III c Aq Insects A mayfly Rhithrogena anomala III c Aq Insects A philopotamid Wormaldia thyria III c caddisfly Aq Insects A rhyacophilid Rhyacophila tricornuta II c caddisfly Aq Insects A water scorpion Nepa apiculata IV c Aq Insects A water strider Limnoporus dissortis IV c Aq Insects Acuminate water Ramphocorixa acuminata IV c boatman Aq Insects Allegheny mayfly Ameletus cryptostimulus IV c Aq Insects Allegheny river cruiser Macromia alleghaniensis IV c Aq Insects Allegheny snaketail Ophiogomphus allegheniensis III c Aq Insects American emerald Cordulia shurtleffi IV c Aq Insects Appalachian jewelwing Calopteryx angustipennis III c Aq Insects Appalachian Rhyacophila appalachia III c rhyacophilid caddisfly Aq Insects Appalachian snaketail Ophiogomphus incurvatus II c alleghaniensis Aq Insects Appalachian stonefly Hansonoperla appalachia II c Aq Insects Banner clubtail Gomphus apomyius IV c Aq Insects Beaverpond baskettail Epitheca canis IV -

The Checklist of Montana Dragonflies & Damselflies
About this Checklist deposit the eggs of further generations. This period River Bluet S c Emma’s Dancer NW,SW,SC o Dragonflies and Damselflies belong to the insect of adult activity is called the Flight Season. Following Enallagma anna M J J A S O N Argia emma M J J A S O N order Odonata, which is split into two suborders: each species is a phenogram [ M J J A S O N ], and Anisoptera – Dragonflies and Zygoptera highlighted in red are the months (May – Nov.) when Familiar Bluet NE,SE c – Damselflies. This checklist includes 53 species of one might expect to see that species during the year. Enallagma civile M J J A S O N Dragonflies (Anisoptera) Dragonflies and 29 species of Damselflies which are Tule Bluet S c known to occur within the state of Montana. Each Species Observed through Oct. 2009 Darners Aeshnidae Enallagma carunculatum M J J A S O N species is listed under its family name and genus. Mosaic Darners Aeshna Common and scientific names are current with those Alkali Bluet S u Damselflies (Zygoptera) Black-tipped Darner NW u set by the Checklist Committee of the Dragonfly Enallagma clausum M J J A S O N Society of the Americas. Aeshna tuberculifera M J J A S O N Broad-winged Damsels Calopterygidae Northern Bluet S c Sedge Darner NW,SW u Jewelwings Calopteryx Enallagma annexum M J J A S O N Distribution Aeshna juncea M J J A S O N To the right of each common name, one or more River Jewelwing NW,SW u Boreal Bluet S c of the following regions will be listed to show the Subarctic Darner NW,SW r Calopteryx aequabilis M J J A S O N Enallagma boreale M J J A S O N approximate distribution of the species within the Aeshna subarctica M J J A S O N Marsh Bluet S c state. -

Telemetry Reveals the Habitat Selected by Immature Dragonflies: Implications for Conservation of the Threatened Dragonfly Leucorrhinia Caudalis (Odonata: Anisoptera)
Journal of Insect Conservation https://doi.org/10.1007/s10841-018-00123-9 ORIGINAL PAPER Telemetry reveals the habitat selected by immature dragonflies: implications for conservation of the threatened dragonfly Leucorrhinia caudalis (Odonata: Anisoptera) Aurélia LeNaour1,2 · Renaud Baeta2 · Eric Sansault2 · Mathieu Deville2 · Sylvain Pincebourde1 Received: 28 July 2018 / Accepted: 20 December 2018 © Springer Nature Switzerland AG 2019 Abstract Determining how species use different habitats during critical phases of their development is one of the crucial challenges that conservation biology meets. However, habitat requirements remain unknown for most species, in particular for the rarest and most threatened which by definition are difficult to study. Here, we used animal-borne telemetry to identify the habitat of the sexually immature adults in the threatened dragonfly Leucorrhinia caudalis. We used an harmonic radar with customized tags fixed on the back of the abdomen of flying immature dragonflies to monitor their position within an area composed of various types of habitats including open areas, forest and water bodies. From 62 tagged individuals, we obtained 23 detec- tions, all within a quite restricted area around the pond of emergence. About 75% of the detections happened in the forest canopy and the individuals were likely positioned at the top of the trees. The relatively low detection rate was probably due to high predation within the study area during the maturation phase in this dragonfly but long-range dispersal cannot be excluded. The use of forest canopy as a maturation habitat is an important knowledge for planning conservation strategies in this endangered species, especially for populations living in areas without any protection status. -

Journal Vol 30 No 2, October 2014
J. Br. Dragonfly Society, Volume 30 No. 2, October 2014 Journal of the CONTENTS DAVID CLARKE – The White-faced Darter (Leucorrhinia British Dragonfly Society dubia Vander Linden) re-introduction project in Cumbria ............................................................................................54 Volume 30 Number 2 October 2014 CHARLES GAUCI – A review of the Odonata of the Maltese Islands ...............................................................................79 ADRIAN J. PARR – Migrant and dispersive dragonflies in Britain during 2012 and 2013........................................110 MARK TYRRELL – The effect of temperature, solar radiation and latitude on the emergence patterns of ‘spring’ species in the south and midlands of Britain .............................125 The aims of the British Dragonfly Society (BDS) are to promote and encourage the study and conservation INSTRUCTIONS TO AUTHORS of Odonata and their natural habitats, especially in the United Kingdom. Authors are asked to study these instructions with care and to prepare their manuscripts accordingly, in order The Journal of the British Dragonfly Society, published twice a year, contains articles on Odonata that have to avoid unnecessary delay in the editing of their manuscripts. been recorded from the United Kingdom and articles on European Odonata written by members of the • Word processed manuscripts may be submitted in electronic form either on disk or by e-mail. Society. • Manuscripts should be one and a half spaced, on one side of the page only and with margins at least 25mm on both sides and top and bottom. Footnotes should be avoided. Articles for publication should be sent to the Editor. Instructions for authors appear inside the back cover. • Use of these terms is acceptable: ‘exuvia’ for cast skin (plural: ‘exuviae’); ‘larva’ (instead of ‘naiad’ or Trustees of the British Dragonfly Society Journal Advisory Panel: ‘nymph’); ‘prolarva’ to designate the first larval instar.