Infrared Spectroscopy Is Widely Used in Both Research and Industry As a Simple and Reliable Technique for Measurement, Quality Control, and Dynamic Measurement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling
Using Visible and Near-Infrared Reflectance Spectroscopy and Differential Scanning Calorimetry to Study Starch, Protein, and Temperature Effects on Bread Staling Feng Xie,1 Floyd E. Dowell,2,3 and Xiuzhi S. Sun1 ABSTRACT Cereal Chem. 81(2):249–254 Starch, protein, and temperature effects on bread staling were inves- retrograded amylose-lipid complex was limited. Two important wave- tigated using visible and near-infrared spectroscopy (NIRS) and differ- lengths, 550 nm and 1,465 nm, were key for NIRS to successfully classify ential scanning calorimetry (DSC). Bread staling was mainly due to the starch-starch (SS) and starch-protein (SP) bread based on different amylopectin retrogradation. NIRS measured amylopectin retrogradation colors and protein contents in SS and SP. Low temperature dramatically accurately in different batches. Three important wavelengths, 970 nm, accelerated the amylopectin retrogradation process. Protein retarded bread 1,155 nm, and 1,395 nm, were associated with amylopectin retrogra- staling, but not as much as temperature. The starch and protein interaction dation. NIRS followed moisture and starch structure changes when was less important than the starch retrogradation. Protein hindered the amylopectin retrograded. The amylose-lipid complex changed little from bread staling process mainly by diluting starch and retarding starch retro- one day after baking. The capability of NIRS to measure changes in the gradation. Bread staling is a complex process that occurs during bread and starch (Osborne and Douglas 1981; Davies and Miller 1988; storage. It is a progressive deterioration of qualities such as taste, Millar et al 1996). Therefore, NIRS has the potential to provide fun- firmness, etc. -

Technical Application Note
Compact Rugged Spectrometers - A Universe of Spectroscopy Systems Technical Application Note Vibrational Spectroscopy: Infrared vs. Raman StellarNet, Inc. Tampa, Fl USA Tony Rizzuto, PhD April 12, 2017 Vibrational spectroscopies are integral in analyzing some of the most fundamentally important processes in physical chemistry: molecular vibrations. While there are many different experimental techniques used to analyze those vibrations, most are variations of the “Big Two,” FTIR and Raman scattering spectroscopies. This article aims to analyze the benefits and drawbacks of each of the “Big Two,” with the hope that it helps one choose the correct technique for one’s research interests. 1. What are the “Big Two?” Fourier Transform Infrared Spectroscopy Raman spectroscopy relies on inelastic (FTIR) is a simple absorption measurement scattering phenomenon that probes the where the detector measures the molecular vibration. Where FTIR will use a absorbance of infrared radiation by the broadband IR source, Raman spectroscopy sample. Each sample will absorb different typically uses a narrow-band, amounts of each frequency resulting in a monochromatic light source in order to “chemical fingerprint” that is the FTIR excite the vibrations of the molecule in your spectrum. sample. 2. Selection Rules: What molecular vibrations are being probed? Imagining a molecular bond vibration with the traditional ball and spring model (Figure 1a) and its resultant harmonic oscillator depiction (Figure 1b) allows us to calculate the selection rules for vibrational transitions to be = 1. However, that does not tell the whole story. In order to truly differentiate FTIR from Raman Spectroscopy, we must think about it on a molecular level. Compact Rugged Spectrometers - A Universe of Spectroscopy Systems Technical Application Note A B Figure 1: A. -

Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification
Comparison Between FTIR and Boehm Titration for Activated Carbon Functional Group Quantification Chad Spreadbury, Regina Rodriguez, and David Mazyck College of Engineering, University of Florida Activated carbon (AC) is a proven effective adsorbent of contaminants in the air and water phases. This effectiveness is due to the large surface area of AC which hosts functional groups. Particularly, oxygen functional groups (e.g. carboxyls, lactones, phenols, carbonyls) have been noted as important in mercury removal. Hence, determining the quantities of these groups is crucial when AC is used for this purpose. Currently, Boehm titration is the most common method for determining the number of these functional groups. However, this test method is susceptible to high user error and require a lengthy procedure and run time. On the other hand, Fourier transform infrared (FTIR) spectroscopy excels at analyzing the functionality of an AC surface. This study investigates whether or not a correlation between Boehm titration and FTIR can be determined using quantitative and qualitative assessment. The results indicate that while using the current methodology for Boehm titrations and FTIR analysis, there is no clear correlation. However, this study does not rule out that a correlation may indeed exist between these test methods that can be elucidated with improved methodology that accounts for the unique physical and chemical characteristics of carbon. INTRODUCTION mercury (Hg0) to create oxidized mercury (Hg2+), which then undergoes chemisorption with the AC surface. ctivated carbon is used globally as an effective Boehm titrations and Fourier transform infrared (FTIR) adsorbent for removing contaminants like mercury spectroscopy are two common testing procedures for A(Hg) from air and water phases. -

Application of High Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared (FTIR) Spectroscopy to Swiss-Typ
Application of High Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared (FTIR) Spectroscopy to Swiss-type Cheese Split Defects THESIS Presented in Partial Fulfillment of the Requirements for the Master of Science Degree in the Graduate School of The Ohio State University By Feifei Hu Graduate Program in Food Science and Technology The Ohio State University 2012 Master's Examination Committee: Dr. W. James Harper, Advisor Dr. Luis E. Rodriguez-Saona Dr. Michael E. Mangino Copyright Feifei Hu 2012 Abstract Splits, slits and cracks are a continuing problem in the US Swiss Cheese industry and cause downgrading of the cheese, bringing economic loss. The defects generally occur during secondary fermentation in cold storage, which is known to be an issue caused by many factors. Many chemical and physical changes can be indicators to help predicting the defects. Various techniques have been applied to test different compounds in cheese. HPLC is one of the reference methods which have been applied in cheese chemistry for the past decades. Its application involves in the qualification and quantification of organic acids, amino acids and peptides, and sugars in dairy products. However, limited research has been conducted on a rapid and easy testing regimen to investigate split defects in Swiss-type cheese. For over fifty years, researchers have investigated the application of Fourier Transform Infrared (FTIR) Spectroscopy in various food samples, including cheeses and other dairy products because FTIR spectroscopy gives fast, simple and comprehensive detection of chemical compounds. There is great potential for applying FTIR spectroscopy in developing a rapid test method to detect and predict the occurrence of split defects. -
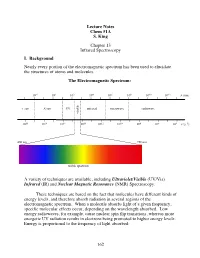
162 Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I
Lecture Notes Chem 51A S. King Chapter 13 Infrared Spectroscopy I. Background Nearly every portion of the electromagnetic spectrum has been used to elucidate the structures of atoms and molecules. The Electromagnetic Spectrum: 10!2 100 102 104 106 108 1010 1012 $ (nm) "!ray X-ray UV infrared microwave radiowave visible 1020 1018 1016 1014 1012 1010 108 106 104 # (s!1) 400 nm 750 nm ! visible spectrum A variety of techniques are available, including Ultraviolet/Visible (UV/Vis) Infrared (IR) and Nuclear Magnetic Resonance (NMR) Spectroscopy. These techniques are based on the fact that molecules have different kinds of energy levels, and therefore absorb radiation in several regions of the electromagnetic spectrum. When a molecule absorbs light of a given frequency, specific molecular effects occur, depending on the wavelength absorbed. Low energy radiowaves, for example, cause nuclear spin flip transitions, whereas more energetic UV radiation results in electrons being promoted to higher energy levels. Energy is proportional to the frequency of light absorbed: 162 Molecular effects associated with different regions of the EM spectrum: Wavelength (!) Energy/mole Molecular effects 10"10 meter gamma rays 106 kcal 10"8 meter X-rays 104 kcal ionization vaccum UV 102 kcal near UV electronic transitions 10"6 meter visible 10 kcal infrared 1 kcal molecular vibrations 10"4 meter (IR) 10"2 kcal 10"2 meter microwave rotational motion 10"4 kcal 0 10 meter radio 10"6 kcal nuclear spin transitions 2 10 meter II. IR Spectroscopy IR radiation causes groups of atoms to vibrate with respect to the bonds that connect them. -

Neutron Vibrational Spectroscopy
Neutron Vibrational Spectroscopy A.J. (Timmy) Ramirez-Cuesta Luke L. Daemen Yongqiang Cheng Spallation Neutron Source Oak Ridge National Laboratory ORNL is managed by UT-Battelle for the US Department of Energy The S(Q,w) Map w=0 Elastic Scattering Diffraction Structural Information 2 "Let there be light" 3 4 How to measure INS (1) Direct Geometry Instrumentation 4000 3500 Direct geometry instruments 3000 ) 1 - m measure Q trajectory is c ( 2500 r e f determined by the angle s n 2000 a r t and energy transfer. y g r 1500 e n Examples: ARCS, CNCS, E HYSPEC, SEQUIOA 1000 500 0 0 10 20 30 Momentum transfer (A-1) ) . U . A ( y t i s n e L t 2 n I Incident neutron beam is 0 10 20 30 40 50 60 70 Energy (meV) s monochromatic t n u o c determining the incident n o r t u Distance energy E1. e N That determines T1. We L1 12000 13000 14000 15000 16000 measure the ToF and we ToF (ms) can work out T2. Resolution is almost constant in units of Ei time 5 VISION: a high throughput spectrometer for neutron vibrational spectroscopy Yongqiang Cheng, Luke Daemen, A.J. (Timmy) Ramirez-Cuesta Spallation Neutron Source, Oak Ridge National Laboratory, Oak Ridge, TN 37931 USA VISION offers users a variety of sample environments How to measure INS (2) Polybenzene nanothreads synthesized at high pressure: single nanothreads VISION is best thought of as and experimental/computational capabilities: the neutron analogue of a Structural inference through modeling of vibrational spectra Indirect Geometry Instrumentation Raman spectrometer. -
Infrared and UV-Visible Time-Resolved Techniques for the Study of Tetrapyrrole-Based Proteins
Infrared and UV-visible time-resolved techniques for the study of tetrapyrrole-based proteins A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy (PhD) in the Faculty of Life Sciences 2013 Henry John Russell Contents List of Tables .......................................................................................................................................................................... 5 List of Figures ........................................................................................................................................................................ 6 Abstract ...................................................................................................................................................................................10 Declaration ............................................................................................................................................................................11 Copyright Statement ........................................................................................................................................................11 Acknowledgements ..........................................................................................................................................................12 Abbreviations and Symbols ..........................................................................................................................................13 Preface and Thesis Structure .......................................................................................................................................16 -

Femtosecond Time-Resolved Observation of Butterfly Vibration In
www.nature.com/scientificreports OPEN Femtosecond time-resolved observation of butterfy vibration in electronically excited Received: 7 June 2017 Accepted: 11 October 2017 o-fuorophenol Published: xx xx xxxx Fengzi Ling1,2, Shuai Li1,2, Xinli Song1,2, Yanmei Wang1,2, Jinyou Long1,2 & Bing Zhang 1,2 The butterfy vibration during the hydrogen tunneling process in electronically excited o-fuorophenol has been visualized in real time by femtosecond time-resolved ion yield spectroscopy coupled with time-resolved photoelectron imaging technique. A coherent superposition of out-of-plane C–F butterfy motions is prepared in the frst excited electronic state (S1). As the C–F bond vibrates with respect to the aromatic ring, the nuclear geometry varies periodically, leading to the corresponding variation in the photoionization channel. By virtue of the more favorable ionization probability from the nonplanar minimum via resonance with the Rydberg states, the evolution of the vibrational wave packet is manifested as a superimposed beat in the parent-ion transient. Moreover, time-resolved photoelectron spectra ofer a direct mapping of the oscillating butterfy vibration between the planar geometry and nonplanar minimum. The beats for the photoelectron peaks originating from the planar geometry are out of phase with those from the nonplanar minimum. Our results provide a physically intuitive and complete picture of the oscillatory fow of energy responsible for the coherent vibrational motion on the excited state surface. Ultrafast molecular vibration is one of the fundamental motions that characterize chemical bonding and decide reaction dynamics at the molecular level1–3. Over the past few decades, frequency-resolved spectroscopies such as infrared and Raman spectra4 and resonant two-photon ionization (R2PI) spectroscopy5 have devoted to pro- viding detailed spectroscopic data about molecular vibration. -

All-Digital Histopathology by Infrared-Optical Hybrid Microscopy
All-digital histopathology by infrared-optical hybrid microscopy Martin Schnella, Shachi Mittala,b, Kianoush Falahkheirkhaha,c, Anirudh Mittala,b, Kevin Yeha,b, Seth Kenkela,d, Andre Kajdacsy-Ballae, P. Scott Carneyf, and Rohit Bhargavaa,b,c,d,g,h,i,1 aBeckman Institute for Advanced Science and Technology, University of Illinois at Urbana–Champaign, Urbana, IL 61801; bDepartment of Bioengineering, University of Illinois at Urbana–Champaign, Urbana, IL 61801; cDepartment of Chemical and Biomolecular Engineering, University of Illinois at Urbana– Champaign, Urbana, IL 61801; dDepartment of Mechanical Science and Engineering, University of Illinois at Urbana–Champaign, Urbana, IL 61801; eDepartment of Pathology, University of Illinois at Chicago, Chicago, IL 60612; fThe Institute of Optics, University of Rochester, Rochester, NY 14620; gCancer Center at Illinois, University of Illinois at Urbana–Champaign, Urbana, IL 61801; hDepartment of Electrical and Computer Engineering, University of Illinois at Urbana–Champaign, Urbana, IL 61801; and iDepartment of Chemistry, University of Illinois at Urbana–Champaign, Urbana, IL 61801 Edited by Christian Huck, University of Innsbruck, Innsbruck, Austria, and accepted by Editorial Board Member John A. Rogers December 20, 2019 (received for review July 19, 2019) Optical microscopy for biomedical samples requires expertise in fidelity imaging in both point-scanning (15) and wide-field (16–19) staining to visualize structure and composition. Midinfrared (mid-IR) modalities, IR pixel sizes are ∼100-fold larger than those easily spectroscopic imaging offers label-free molecular recording and achieved in visible microscopy, and data acquisition speed is still virtual staining by probing fundamental vibrational modes of three to four orders of magnitude slower than in visible imaging molecular components. -

And Raman Spectroscopy IR and Raman Spectroscopy Measure the Energy Difference of Vibrational Energy Levels in Molecules, They Are Energy Sensitive Methods
Infrared (IR) and Raman Spectroscopy IR and Raman spectroscopy measure the energy difference of vibrational energy levels in molecules, they are energy sensitive methods. They are based on interactions of electromagnetic radiation and material but the main differences between these two spectroscopic techniques are the physical effects. For infrared spectroscopy the absorption process of polychromatic IR radiation due to the molecule cause a change in the dipole moment of the molecule and the molecule will be translated from a ground state to a vibrational excited state. Whereas the monochromatic radiation, for Raman spectroscopy, is inelastic scattered at the electron shell of the molecule which changes the molecular polarizability and the radiation will be shifted in frequency (light source: UV, VIS or NIR lasers). The “Raman effect” was discovered by the Indian physicist, C. V. Raman inn 1928. Selection rules: Whether or not a vibrational mode is active or inactive for one of these complementary (inversely related) techniques depends on the symmetry of the molecules. For example, homonuclear diatomic molecules are not IR active, because they have no dipole moment, but they are Raman active. Because of the stretching and contraction of the bond changes the interactions between the electrons and nuclei, this causes a change of polarizability. For highly symmetric polyatomic molecules possessing a center of inversion, the bands are IR active (Raman inactive) for asymmetric vibrations to i and for symmetric vibrations to i the bands are Raman active (IR inactive). A mode can be IR active, Raman inactive and vice-versa however not at the same time. This fact is named as mutual exclusion rule. -

Fourier Transform Infrared Spectroscopy
FOURIER TRANSFORM INFRARED SPECTROSCOPY Purpose and Scope: This document describes the procedures and policies for using the MSE Bruker FTIR Spectrometer. The scope of this document is to establish user procedures. Instrument maintenance and repair are outside the scope of this document. Responsibilities: This document is maintained by the department Lab manager. The Lab Manager is responsible for general maintenance and for arranging repair when necessary. If you feel that the instrument is in need of repair or is not operating correctly, please notify the Lab Manager immediately. The Lab Manger will operate the instruments according to the procedures set down in this document and will provide instruction and training to users within the department. Users are responsible for using the instrument described according to these procedures. These procedures assume that the user has had at least one training session. Warnings and precautions: • When using the EGA-TGA Cell remember that it is hot. The cell and the line from the TGA to the cell are operated at 200C. • When using the EGA-TGA cell please remember to turn off the heater by pressing the down arrow on the controller until the set point reads “off”. You will be shown how to do this during training. • User are NOT cleared to change out fixtures. Only lab personnel are allowed to install or remove the ATR, the pellet holder, the EGA-TGA Cell or any other fixtures. We recommend that you notify lab personnel the day before your scheduled measurement to ensure it is ready. • For measuring KBr pellets: Users are expected to make/have their own pellet equipment. -

What-Is -Ft-Ir.Pdf
What is FT‐IR? Infrared (IR) spectroscopy is a chemical analytical technique, which measures the infrared intensity versus wavelength (wavenumber) of light. Based upon the wavenumber, infrared light can be categorized as far infrared (4 ~ 400cm‐1), mid infrared (400 ~ 4,000cm‐1) and near infrared (4,000 ~ 14,000cm‐1). Infrared spectroscopy detects the vibration characteristics of chemical functional groups in a sample. When an infrared light interacts with the matter, chemical bonds will stretch, contract and bend. As a result, a chemical functional group tends to adsorb infrared radiation in a specific wavenumber range regardless of the structure of the rest of the molecule. For example, the C=O stretch of a carbonyl group appears at around 1700cm‐1 in a variety of molecules. Hence, the correlation of the band wavenumber position with the chemical structure is used to identify a functional group in a sample. The wavenember positions where functional groups adsorb are consistent, despite the effect of temperature, pressure, sampling, or change in the molecule structure in other parts of the molecules. Thus the presence of specific functional groups can be monitored by these types of infrared bands, which are called group wavenumbers. The early‐stage IR instrument is of the dispersive type, which uses a prism or a grating monochromator. The dispersive instrument is characteristic of a slow scanning. A Fourier Transform Infrared (FTIR) spectrometer obtains infrared spectra by first collecting an interferogram of a sample signal with an interferometer, which measures all of infrared frequencies simultaneously. An FTIR spectrometer acquires and digitizes the interferogram, performs the FT function, and outputs the spectrum.